Intestinal bile acid sequestration improves glucose control by stimulating hepatic miR-182-5p in type 2 diabetes
- PMID: 30160993
- PMCID: PMC6415711
- DOI: 10.1152/ajpgi.00238.2018
Intestinal bile acid sequestration improves glucose control by stimulating hepatic miR-182-5p in type 2 diabetes
Abstract
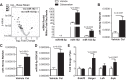
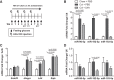
Colesevelam is a bile acid sequestrant approved to treat both hyperlipidemia and type 2 diabetes, but the mechanism for its glucose-lowering effects is not fully understood. The aim of this study was to investigate the role of hepatic microRNAs (miRNAs) as regulators of metabolic disease and to investigate the link between the cholesterol and glucose-lowering effects of colesevelam. To quantify the impact of colesevelam treatment in rodent models of diabetes, metabolic studies were performed in Zucker diabetic fatty (ZDF) rats and db/db mice. Colesevelam treatments significantly decreased plasma glucose levels and increased glycolysis in the absence of changes to insulin levels in ZDF rats and db/db mice. High-throughput sequencing and real-time PCR were used to quantify hepatic miRNA and mRNA changes, and the cholesterol-sensitive miR-96/182/183 cluster was found to be significantly increased in livers from ZDF rats treated with colesevelam compared with vehicle controls. Inhibition of miR-182 in vivo attenuated colesevelam-mediated improvements to glycemic control in db/db mice. Hepatic expression of mediator complex subunit 1 (MED1), a nuclear receptor coactivator, was significantly decreased with colesevelam treatments in db/db mice, and MED1 was experimentally validated to be a direct target of miR-96/182/183 in humans and mice. In summary, these results support that colesevelam likely improves glycemic control through hepatic miR-182-5p, a mechanism that directly links cholesterol and glucose metabolism. NEW & NOTEWORTHY Colesevelam lowers systemic glucose levels in Zucker diabetic fatty rats and db/db mice and increases hepatic levels of the sterol response element binding protein 2-responsive microRNA cluster miR-96/182/183. Inhibition of miR-182 in vivo reverses the glucose-lowering effects of colesevelam in db/db mice. Mediator complex subunit 1 (MED1) is a novel, direct target of the miR-96/182/183 cluster in mice and humans.
Keywords: bile acid sequestrants; cholesterol; glucose metabolism; microRNAs; sterol-regulatory element binding protein 2.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Allen RM, Zhao S, Ramirez Solano MA, Zhu W, Michell DL, Wang Y, Shyr Y, Sethupathy P, Linton MF, Graf GA, Sheng Q, Vickers KC. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J Extracell Vesicles 7: 1506198, 2018. doi: 10.1080/20013078.2018.1506198. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

