Noninvasive allergic sinus congestion and resolution assessments using microcomputed tomography imaging
- PMID: 30161008
- PMCID: PMC6295483
- DOI: 10.1152/japplphysiol.00980.2017
Noninvasive allergic sinus congestion and resolution assessments using microcomputed tomography imaging
Abstract
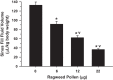
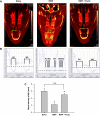
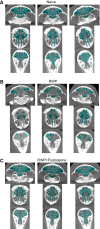
Sinus congestion resultant of allergic rhinosinusitis is associated with development and worsening of asthma and can result in difficulty breathing, headaches, and missed days of school and work. Quantification of sinus congestion is important in the understanding of allergic rhinosinusitis and the development of new drugs for its treatment. Noninvasive microcomputed tomography (micro-CT) was investigated in a guinea pig model of allergic rhinosinusitis to determine its utility to determine accurately the degree of sinus congestion and resolution with anti-inflammatory drug administration. Three-dimensional sinus air-space volume, two-dimensional sinus width, sinus image air-space area, and sinus image sinus perimeter were measured in guinea pigs administered ragweed pollen (RWP), intranasally (i.n.), followed by administration of fluticasone, i.n. To determine their relative accuracy in assessing sinus congestion, the micro-CT image results were compared with the "gold-standard" method of sinus fluid fill-volume (SFFV) measurements. As measured by SFFV method, RWP increased sinus congestion in a RWP concentration-dependent fashion, approaching near-total sinus blockage with concentrations ≥22 µg of RWP. At this level of congestion, fluticasone (25-100 µg) progressively decreased sinus congestion in a concentration-dependent fashion. The noninvasive micro-CT methods were found to accurately determine the amount of sinus congestion and resolution, with patterns of increases and decreases of congestion that were nearly identical to the SFFV method. We conclude that noninvasive micro-CT measurements of allergic sinus congestion can be useful as an investigative tool in the assessment of congestion intensity and the development of new drug therapies for its treatment. NEW & NOTEWORTHY Allergic rhinosinusitis afflicts significant portions of the world population, resulting in loss of work productivity and decreased quality of life. Thus the development of methodological approaches, which incorporate accurate and reproducible noninvasive assessments of sinus congestion, are desirable. Microcomputed tomography of the guinea pig sinuses offers a noninvasive evaluation tool in an animal model of IgE-dependent allergy similar to that in humans, with potential relevance toward development of therapeutics for human sinus diseases.
Keywords: allergic sinus inflammation; guinea pig; micro-CT; ragweed pollen; rhinosinusitis.
Conflict of interest statement
W. C. Spear and B. T. Ameredes received grant funding from Alcon Research, Ltd. and the Forest Research Institute in support of a portion of this work.
Figures







References
-
- Bachert C, Marquardt U, Korte M. IgE-positive mast cells play a central role in nasal allergic disease. Am J Rhinol 4: 215–219, 1990. doi:10.2500/105065890782009299. - DOI
-
- Belvisi MG, Birrell MA, Khalid S, Wortley MA, Dockry R, Coote J, Holt K, Dubuis E, Kelsall A, Maher SA, Bonvini S, Woodcock A, Smith JA. Neurophenotypes in airway diseases. Insights from translational cough studies. Am J Respir Crit Care Med 193: 1364–1372, 2016. doi:10.1164/rccm.201508-1602OC. - DOI - PMC - PubMed
-
- Bousquet J, Bachert C, Bernstein J, Canonica GW, Carr W, Dahl R, Demoly P, Devillier P, Hellings P, Fokkens W, Klimek L, Lieberman P, Meltzer E, Price D, Ryan D, Wahn U. Advances in pharmacotherapy for the treatment of allergic rhinitis; MP29-02 (a novel formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) fills the gaps. Expert Opin Pharmacother 16: 913–928, 2015. doi:10.1517/14656566.2015.1020789. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

