Prevalence of Novel Candidate Sjogren Syndrome Autoantibodies in the Dry Eye Assessment and Management (DREAM) Study
- PMID: 30161055
- PMCID: PMC6173620
- DOI: 10.1097/ICO.0000000000001714
Prevalence of Novel Candidate Sjogren Syndrome Autoantibodies in the Dry Eye Assessment and Management (DREAM) Study
Abstract
Purpose: To evaluate the prevalence of novel candidate Sjogren syndrome (SS) autoantibodies [salivary protein-1 (SP-1), parotid secretory protein, carbonic anhydrase 6] in the DRy Eye Assessment and Management (DREAM) cohort, a study evaluating the effectiveness of omega-3 fatty acid supplements for the treatment of dry eye.
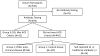
Methods: Participants underwent ocular surface examinations and serological testing for traditional and novel SS autoantibodies. Dry eye assessment and management participants were categorized into the following 3 groups: 1) no history of SS or other autoimmune diseases and negative traditional SS autoantibodies (n = 352); 2) no history of SS but a history of other autoimmune diseases (n = 66); and 3) those who met the 2012 American College of Rheumatology SS classification criteria (n = 52).
Results: Eleven percent had a history of SS, and 6% of those without a history of SS most likely had undiagnosed SS. The SS group had a higher prevalence of SP-1 autoantibodies than the group without SS or other autoimmune diseases (33% vs. 19%; P = 0.02) but had no difference in carbonic anhydrase 6 (P = 0.31) or parotid secretory protein autoantibodies (P = 0.33). Participants who were positive for the traditional autoantibodies alone or positive for both traditional and novel autoantibodies had the highest scores for corneal (P = 0.002) and conjunctival staining (P < 0.001).
Conclusions: Data from this multicenter, prospective study demonstrated that one of the novel candidate autoantibodies, SP-1, is associated with underlying SS and that novel autoantibodies may be associated with worse ocular surface disease. Future longitudinal studies are needed to evaluate their utility in screening patients with dry eye for SS.
Conflict of interest statement
Figures
References
-
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I Arthritis Rheum. 2008;58:15–25. - PubMed
-
- Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337–44. - PubMed
-
- Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med. 2004;164:1275–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous