Dissecting the mechanism of action of actinoporins. Role of the N-terminal amphipathic α-helix in membrane binding and pore activity of sticholysins I and II
- PMID: 30161192
- PMCID: PMC6117003
- DOI: 10.1371/journal.pone.0202981
Dissecting the mechanism of action of actinoporins. Role of the N-terminal amphipathic α-helix in membrane binding and pore activity of sticholysins I and II
Abstract
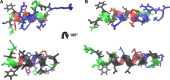
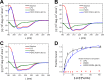
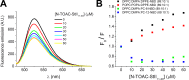
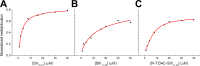
Actinoporins sticholysin I and sticholysin II (St I, St II) are proposed to lyse model and biomembranes via toroidal pore formation by their N-terminal domain. Based on the hypothesis that peptide fragments can reproduce the structure and function of this domain, the behavior of peptides containing St I residues 12-31 (StI12-31), St II residues 11-30 (StII11-30), and its TOAC-labeled analogue (N-TOAC-StII11-30) was examined. Molecular modeling showed a good match with experimental structures, indicating amphipathic α-helices in the same regions as in the toxins. CD spectra revealed that the peptides were essentially unstructured in aqueous solution, acquiring α-helical conformation upon interaction with micelles and large unilamellar vesicles (LUV) of variable lipid composition. Fluorescence quenching studies with NBD-containing lipids indicated that N-TOAC-StII11-30's nitroxide moiety is located in the membranes polar head group region. Pyrene-labeled phospholipid inter-leaflet redistribution suggested that the peptides form toroidal pores, according to the mechanism of action proposed for the toxins. Binding occurred only to negatively charged LUV, indicating the importance of electrostatic interactions; in contrast the peptides bound to both negatively charged and zwitterionic micelles, pointing to a lesser influence of these interactions. In addition, differences between bilayers and micelles in head group packing and in curvature led to differences in peptide-membrane interaction. We propose that the peptides topography in micelles resembles that of the toxins in the toroidal pore. The peptides mimicked the toxins permeabilizing activity, St II peptides being more effective than StI12-31. To our knowledge, this is the first demonstration that differences in the toxins N-terminal amphipathic α-helix play a role in the difference between St I and St II activities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Extension of sticholysins N-terminal α-helix signals membrane lipids to acquire curvature for toroidal pore formation.Biochem Biophys Res Commun. 2025 Jan;742:151071. doi: 10.1016/j.bbrc.2024.151071. Epub 2024 Nov 27. Biochem Biophys Res Commun. 2025. PMID: 39657352
-
Binding of sea anemone pore-forming toxins sticholysins I and II to interfaces--modulation of conformation and activity, and lipid-protein interaction.Chem Phys Lipids. 2003 Jan;122(1-2):97-105. doi: 10.1016/s0009-3084(02)00181-0. Chem Phys Lipids. 2003. PMID: 12598041
-
Sticholysins, two pore-forming toxins produced by the Caribbean Sea anemone Stichodactyla helianthus: their interaction with membranes.Toxicon. 2009 Dec 15;54(8):1135-47. doi: 10.1016/j.toxicon.2009.02.022. Epub 2009 Mar 4. Toxicon. 2009. PMID: 19268489 Review.
-
Effects of lipid composition on membrane permeabilization by sticholysin I and II, two cytolysins of the sea anemone Stichodactyla helianthus.Biophys J. 2001 Jun;80(6):2761-74. doi: 10.1016/S0006-3495(01)76244-3. Biophys J. 2001. PMID: 11371451 Free PMC article.
-
Molecular mechanism of sphingomyelin-specific membrane binding and pore formation by actinoporins.Adv Exp Med Biol. 2010;677:106-15. Adv Exp Med Biol. 2010. PMID: 20687484 Review.
Cited by
-
Functional and Structural Variation among Sticholysins, Pore-Forming Proteins from the Sea Anemone Stichodactyla helianthus.Int J Mol Sci. 2020 Nov 24;21(23):8915. doi: 10.3390/ijms21238915. Int J Mol Sci. 2020. PMID: 33255441 Free PMC article. Review.
-
Membrane Remodeling by the Lytic Fragment of SticholysinII: Implications for the Toroidal Pore Model.Biophys J. 2019 Nov 5;117(9):1563-1576. doi: 10.1016/j.bpj.2019.09.018. Epub 2019 Sep 20. Biophys J. 2019. PMID: 31587828 Free PMC article.
-
Uprising Unconventional Nanobiomaterials: Peptoid Nanosheets as a Multi-Modular Platform for Advanced Biological Studies.Small. 2025 Mar;21(9):e2406128. doi: 10.1002/smll.202406128. Epub 2024 Dec 1. Small. 2025. PMID: 39618020 Free PMC article. Review.
-
Dimerization of Antimicrobial Peptides: A Promising Strategy to Enhance Antimicrobial Peptide Activity.Protein Pept Lett. 2019;26(2):98-107. doi: 10.2174/0929866526666190102125304. Protein Pept Lett. 2019. PMID: 30605048 Free PMC article. Review.
-
Membrane activity of two short Trp-rich amphipathic peptides.Biochim Biophys Acta Biomembr. 2020 Jul 1;1862(7):183280. doi: 10.1016/j.bbamem.2020.183280. Epub 2020 Mar 24. Biochim Biophys Acta Biomembr. 2020. PMID: 32220553 Free PMC article.
References
-
- de los Ríos V, Mancheño JM, Martinez Del Pozo A, Alfonso C, Rivas G, Oñaderra M, et al. Sticholysin II, a cytolysin from the sea anemone Stichodactyla helianthus, is a monomer-tetramer associating protein. FEBS Lett. 1999; 455:27–30. - PubMed
-
- Lanio ME, Morera V, Alvarez C, Tejuca M, Gomez T, Pazos F, et al. Purification and characterization of two hemolysins from Stichodactyla helianthus. Toxicon 2001; 39:187–194. - PubMed
-
- Huerta V, Morera V, Guanche Y, Chinea G, Gonzalez LJ, Betancourt L, et al. Primary structure of two cytolysin isoforms from Stichodactyla helianthus differing in their hemolytic activity. Toxicon 2001; 39:1253–1256. - PubMed
-
- Mancheño JM, Benito JM, Martinez-Ripoll M, Gavilanes JG, Hermoso JA. Crystal and electron microscopy structures of Sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure 2003; 11:1319–1328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

