Neuroprotective exendin-4 enhances hypothermia therapy in a model of hypoxic-ischaemic encephalopathy
- PMID: 30165597
- PMCID: PMC6158761
- DOI: 10.1093/brain/awy220
Neuroprotective exendin-4 enhances hypothermia therapy in a model of hypoxic-ischaemic encephalopathy
Abstract
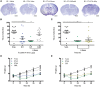
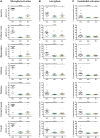
Hypoxic-ischaemic encephalopathy remains a global health burden. Despite medical advances and treatment with therapeutic hypothermia, over 50% of cooled infants are not protected and still develop lifelong neurodisabilities, including cerebral palsy. Furthermore, hypothermia is not used in preterm cases or low resource settings. Alternatives or adjunct therapies are urgently needed. Exendin-4 is a drug used to treat type 2 diabetes mellitus that has also demonstrated neuroprotective properties, and is currently being tested in clinical trials for Alzheimer's and Parkinson's diseases. Therefore, we hypothesized a neuroprotective effect for exendin-4 in neonatal neurodisorders, particularly in the treatment of neonatal hypoxic-ischaemic encephalopathy. Initially, we confirmed that the glucagon like peptide 1 receptor (GLP1R) was expressed in the human neonatal brain and in murine neurons at postnatal Day 7 (human equivalent late preterm) and postnatal Day 10 (term). Using a well characterized mouse model of neonatal hypoxic-ischaemic brain injury, we investigated the potential neuroprotective effect of exendin-4 in both postnatal Day 7 and 10 mice. An optimal exendin-4 treatment dosing regimen was identified, where four high doses (0.5 µg/g) starting at 0 h, then at 12 h, 24 h and 36 h after postnatal Day 7 hypoxic-ischaemic insult resulted in significant brain neuroprotection. Furthermore, neuroprotection was sustained even when treatment using exendin-4 was delayed by 2 h post hypoxic-ischaemic brain injury. This protective effect was observed in various histopathological markers: tissue infarction, cell death, astrogliosis, microglial and endothelial activation. Blood glucose levels were not altered by high dose exendin-4 administration when compared to controls. Exendin-4 administration did not result in adverse organ histopathology (haematoxylin and eosin) or inflammation (CD68). Despite initial reduced weight gain, animals restored weight gain following end of treatment. Overall high dose exendin-4 administration was well tolerated. To mimic the clinical scenario, postnatal Day 10 mice underwent exendin-4 and therapeutic hypothermia treatment, either alone or in combination, and brain tissue loss was assessed after 1 week. Exendin-4 treatment resulted in significant neuroprotection alone, and enhanced the cerebroprotective effect of therapeutic hypothermia. In summary, the safety and tolerance of high dose exendin-4 administrations, combined with its neuroprotective effect alone or in conjunction with clinically relevant hypothermia make the repurposing of exendin-4 for the treatment of neonatal hypoxic-ischaemic encephalopathy particularly promising.
Figures






Similar articles
-
Targeting Persistent Neuroinflammation after Hypoxic-Ischemic Encephalopathy-Is Exendin-4 the Answer?Int J Mol Sci. 2022 Sep 5;23(17):10191. doi: 10.3390/ijms231710191. Int J Mol Sci. 2022. PMID: 36077587 Free PMC article. Review.
-
Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model.Brain. 2013 Jan;136(Pt 1):90-105. doi: 10.1093/brain/aws285. Epub 2012 Nov 26. Brain. 2013. PMID: 23183236
-
Hypothermia Is Neuroprotective after Severe Hypoxic-Ischaemic Brain Injury in Neonatal Rats Pre-Exposed to PAM3CSK4.Dev Neurosci. 2018;40(3):189-197. doi: 10.1159/000487798. Epub 2018 Jun 1. Dev Neurosci. 2018. PMID: 29860252
-
The duration of hypothermia affects short-term neuroprotection in a mouse model of neonatal hypoxic ischaemic injury.PLoS One. 2018 Jul 3;13(7):e0199890. doi: 10.1371/journal.pone.0199890. eCollection 2018. PLoS One. 2018. PMID: 29969470 Free PMC article.
-
New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy.Eur J Pediatr. 2022 Mar;181(3):875-887. doi: 10.1007/s00431-021-04320-8. Epub 2021 Nov 24. Eur J Pediatr. 2022. PMID: 34820702 Free PMC article. Review.
Cited by
-
Neuroprotective Effects of Diabetes Drugs for the Treatment of Neonatal Hypoxia-Ischemia Encephalopathy.Front Cell Neurosci. 2020 May 6;14:112. doi: 10.3389/fncel.2020.00112. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32435185 Free PMC article. Review.
-
Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury.Int J Biol Sci. 2022 Jan 16;18(4):1328-1346. doi: 10.7150/ijbs.68974. eCollection 2022. Int J Biol Sci. 2022. PMID: 35280691 Free PMC article.
-
Structural Changes Observed in the Piriform Cortex in a Rat Model of Pre-motor Parkinson's Disease.Front Cell Neurosci. 2018 Dec 10;12:479. doi: 10.3389/fncel.2018.00479. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618629 Free PMC article.
-
Crocin enhances hypothermia therapy in hypoxic ischemia-induced brain injury in mice.Acta Neurol Belg. 2021 Apr;121(2):429-436. doi: 10.1007/s13760-019-01198-0. Epub 2019 Jul 31. Acta Neurol Belg. 2021. PMID: 31367946
-
Targeting Persistent Neuroinflammation after Hypoxic-Ischemic Encephalopathy-Is Exendin-4 the Answer?Int J Mol Sci. 2022 Sep 5;23(17):10191. doi: 10.3390/ijms231710191. Int J Mol Sci. 2022. PMID: 36077587 Free PMC article. Review.
References
-
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 2016; 21: 802–18. - PubMed
-
- Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain 2013b; 136: 374–84. - PubMed
-
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J et al. . Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 2008; 86: 326–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

