Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells
- PMID: 30165855
- PMCID: PMC6117929
- DOI: 10.1186/s12931-018-0861-5
Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells
Abstract
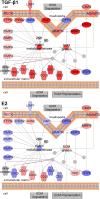
Background: Sex differences in idiopathic pulmonary fibrosis (IPF) suggest a protective role for estrogen (E2); however, mechanistic studies in animal models have produced mixed results. Reports using cell lines have investigated molecular interactions between transforming growth factor beta1 (TGF-β1) and estrogen receptor (ESR) pathways in breast, prostate, and skin cells, but no such interactions have been described in human lung cells. To address this gap in the literature, we investigated a role for E2 in modulating TGF-β1-induced signaling mechanisms and identified novel pathways impacted by estrogen in bronchial epithelial cells.
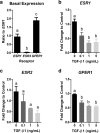
Methods: We investigated a role for E2 in modulating TGF-β1-induced epithelial to mesenchymal transition (EMT) in bronchial epithelial cells (BEAS-2Bs) and characterized the effect of TGF-β1 on ESR mRNA and protein expression in BEAS-2Bs. We also quantified mRNA expression of ESRs in lung tissue from individuals with IPF and identified potential downstream targets of E2 signaling in BEAS-2Bs using RNA-Seq and gene set enrichment analysis.
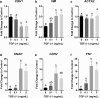
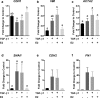
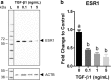
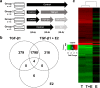
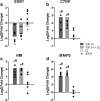
Results: E2 negligibly modulated TGF-β1-induced EMT; however, we report the novel observation that TGF-β1 repressed ESR expression, most notably estrogen receptor alpha (ESR1). Results of the RNA-Seq analysis showed that TGF-β1 and E2 inversely modulated the expression of several genes involved in processes such as extracellular matrix (ECM) turnover, airway smooth muscle cell contraction, and calcium flux regulation. We also report that E2 specifically modulated the expression of genes involved in chromatin remodeling pathways and that this regulation was absent in the presence of TGF-β1.
Conclusions: Collectively, these results suggest that E2 influences unexplored pathways that may be relevant to pulmonary disease and highlights potential roles for E2 in the lung that may contribute to sex-specific differences.
Keywords: Estrogen; Estrogen receptor; Fibrosis; Lung; Transforming growth factor beta1.
Conflict of interest statement
Ethics approval and consent to participate
The human lung tissue samples used in this study were a kind gift from Dr. Andrew Bryant. The deidentified, explanted lung tissue was obtained from subjects undergoing lung transplant for IPF and from lungs rejected for transplant from normal controls per the National Institutes of Health Lung Tissue Research Consortium (protocol no. 14-99-0011). The protocol for collection of lung tissue samples, and subsequent studies, were approved by the institutional review board at Vanderbilt University and the University of Florida (30).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Tannic acid attenuates TGF-β1-induced epithelial-to-mesenchymal transition by effectively intervening TGF-β signaling in lung epithelial cells.J Cell Physiol. 2018 Mar;233(3):2513-2525. doi: 10.1002/jcp.26127. Epub 2017 Aug 30. J Cell Physiol. 2018. PMID: 28771711
-
Family with sequence similarity 13 member A mediates TGF-β1-induced EMT in small airway epithelium of patients with chronic obstructive pulmonary disease.Respir Res. 2021 Jul 1;22(1):192. doi: 10.1186/s12931-021-01783-z. Respir Res. 2021. PMID: 34210319 Free PMC article.
-
TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT).Respir Res. 2005 Jun 9;6(1):56. doi: 10.1186/1465-9921-6-56. Respir Res. 2005. PMID: 15946381 Free PMC article.
-
Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β1 in A549, NCI-H441, and Calu-3 cells: Development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis.J Pharmacol Toxicol Methods. 2017 Jul;86:19-27. doi: 10.1016/j.vascn.2017.02.023. Epub 2017 Mar 1. J Pharmacol Toxicol Methods. 2017. PMID: 28259823
-
Transforming growth factor-beta 1 regulates lung epithelial barrier function and fluid transport.Am J Physiol Lung Cell Mol Physiol. 2003 Dec;285(6):L1190-1. doi: 10.1152/ajplung.00230.2003. Am J Physiol Lung Cell Mol Physiol. 2003. PMID: 14604849 Review. No abstract available.
Cited by
-
Evaluation of Proteasome Inhibitors in the Treatment of Idiopathic Pulmonary Fibrosis.Cells. 2022 May 4;11(9):1543. doi: 10.3390/cells11091543. Cells. 2022. PMID: 35563849 Free PMC article. Review.
-
Sex Steroids Effects on Asthma: A Network Perspective of Immune and Airway Cells.Cells. 2022 Jul 19;11(14):2238. doi: 10.3390/cells11142238. Cells. 2022. PMID: 35883681 Free PMC article. Review.
-
MicroRNA let-7 Downregulates Ligand-Independent Estrogen Receptor-mediated Male-Predominant Pulmonary Fibrosis.Am J Respir Crit Care Med. 2019 Nov 15;200(10):1246-1257. doi: 10.1164/rccm.201903-0508OC. Am J Respir Crit Care Med. 2019. PMID: 31291549 Free PMC article.
-
Collagen Triple Helix Repeat Containing 1 Deficiency Protects Against Airway Remodeling and Inflammation in Asthma Models In Vivo and In Vitro.Inflammation. 2023 Jun;46(3):925-940. doi: 10.1007/s10753-022-01781-3. Epub 2023 Jan 14. Inflammation. 2023. PMID: 36640227
-
Toxicity of microplastic fibers containing azobenzene disperse dyes to human lung epithelial cells cultured at an air-liquid interface.J Hazard Mater. 2024 Dec 5;480:136280. doi: 10.1016/j.jhazmat.2024.136280. Epub 2024 Oct 28. J Hazard Mater. 2024. PMID: 39515142
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

