Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy
- PMID: 30166439
- PMCID: PMC6205228
- DOI: 10.1126/science.aau1549
Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy
Abstract
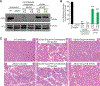
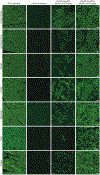
Mutations in the gene encoding dystrophin, a protein that maintains muscle integrity and function, cause Duchenne muscular dystrophy (DMD). The deltaE50-MD dog model of DMD harbors a mutation corresponding to a mutational "hotspot" in the human DMD gene. We used adeno-associated viruses to deliver CRISPR gene editing components to four dogs and examined dystrophin protein expression 6 weeks after intramuscular delivery (n = 2) or 8 weeks after systemic delivery (n = 2). After systemic delivery in skeletal muscle, dystrophin was restored to levels ranging from 3 to 90% of normal, depending on muscle type. In cardiac muscle, dystrophin levels in the dog receiving the highest dose reached 92% of normal. The treated dogs also showed improved muscle histology. These large-animal data support the concept that, with further development, gene editing approaches may prove clinically useful for the treatment of DMD.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
CRISPR therapy shows promise in Duchenne muscular dystrophy.Nat Rev Neurol. 2018 Nov;14(11):632-633. doi: 10.1038/s41582-018-0078-8. Nat Rev Neurol. 2018. PMID: 30237553 No abstract available.
-
Gene editing for Duchenne muscular dystrophy.Nat Med. 2018 Oct;24(10):1491. doi: 10.1038/s41591-018-0225-1. Nat Med. 2018. PMID: 30297891 No abstract available.
-
CRISPR alleviates muscular dystrophy in dogs.Nat Biomed Eng. 2018 Nov;2(11):795-796. doi: 10.1038/s41551-018-0320-0. Nat Biomed Eng. 2018. PMID: 31015617 No abstract available.
References
-
- O’Brien KF, Kunkel LM, Mol. Genet. Metab 74, 75–88 (2001). - PubMed
-
- Guiraud S et al. , Annu. Rev. Genomics Hum. Genet 16, 281–308 (2015). - PubMed
-
- Campbell KP, Kahl SD, Nature 338, 259–262 (1989). - PubMed
-
- Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG,Campbell KP, Nature 345, 315–319 (1990). - PubMed
-
- Muntoni F, Torelli S, Ferlini A, Lancet Neurol 2, 731–740 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

