THE JEREMIAH METZGER LECTURE NOVEL THERAPEUTIC STRATEGIES OF ALLERGIC AND IMMUNOLOGIC DISORDERS
- PMID: 30166721
- PMCID: PMC6116601
THE JEREMIAH METZGER LECTURE NOVEL THERAPEUTIC STRATEGIES OF ALLERGIC AND IMMUNOLOGIC DISORDERS
Abstract
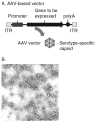
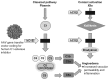
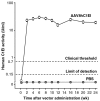
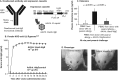
Advances in understanding the immunological basis and mechanisms underlying allergic and immunologic disorders have led to effective but costly long-term and repetitive biologic therapies. Gene therapy is a rapidly advancing technology, in which a single administration of an adeno-associated virus encoding the therapeutic protein or monoclonal antibody may provide effective long-term therapy for allergic and immunologic disorders. In this review, we summarize the recent studies from our laboratory developing gene therapy strategies to treat hereditary angioedema and peanut allergy. The unraveling of the pathogenesis of immune-based disorders, including hereditary deficiencies of components of the immune system and allergic disorders, has led to the development of therapies using parenteral administration of recombinant proteins or monoclonal antibodies (1). While many of these therapies are highly effective, they are limited by the half-life of the therapeutic protein or antibody, requiring repetitive administration of days to weeks (2-15). The focus of recent work in our laboratory has been to solve this problem by substituting protein/monoclonal antibody administration with gene therapy, where current technology allows for a single administration of the gene coding for a protein or antibody to provide persistent expression of effective levels of the therapeutic protein or antibody. Gene therapy is a drug delivery platform which uses genetic material, usually in the form of coding exons of the therapeutic gene, to correct, compensate for, or prevent the development of an abnormal phenotype (16). Originally conceptualized as a strategy to treat rare hereditary disorders, gene therapy is being developed for a wide range of human disorders, including common acquired conditions (17-20). In this review, we will describe how we have adopted gene therapy technology to develop therapies for immune-related disorders, using as examples hereditary angioedema, an inherited autosomal dominant disorder, and peanut allergy, a common acquired allergic disorder.
Conflict of interest statement
Potential Conflicts of Interest: Cornell University has licensed the patent disclosure relating to gene therapy for C1EI deficiency and peanut allergy to Adverum Biotechnologies. Dr. Crystal holds stock and is a consultant to Adverum. Drs. Pagovich and Crystal are inventors on relevant patent disclosures. These studies were supported, in part, by NIH R03 AI22040 and Adverum Biotechnologies.
Figures
References
-
- Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22:868–76. - PubMed
-
- CSL Behring, HAEGARDA, C1 esterase inhibitor subcutaneous (human) 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/... . Accessed January 18, 2018.
-
- CSL Behring. Berinert, C1 Esterase inhibitor, human. 2018. Available at: http://www.berinert.com/prescribing-information.aspx . Accessed January 18, 2018.
-
- Shire. CINRYZE. 2016. Available at: https://www.cinryze.com/hcp . Accessed January 18, 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
