Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles
- PMID: 30166902
- PMCID: PMC6111123
- DOI: 10.1016/j.jsps.2017.06.001
Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles
Abstract
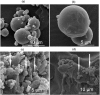
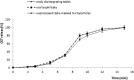
Orally disintegrating tablets and oral lyophilisates are novel attractive dosage forms that disintegrate or dissolve in the buccal cavity within seconds without necessity of drinking. The major limitation in designing of these dosage forms is unpleasant taste of the drug substance. Cetirizine dihydrochloride is a H1-antihistamine substance indicated for the treatment of allergy. It is characterized by extremely bitter taste, therefore in order to deliver cetirizine dihydrochloride using orodispersible formulations, effective taste-masking is required. The aim of this study was to investigate whether microparticles containing cetirizine dihydrochloride could be successfully used to formulate orally disintegrating tablets by direct compression method and oral lyophilisates by freeze-drying process. Taste masking of cetirizine dihydrochloride was achieved by the spray-drying technique using Eudragit® E PO as the drug agent carrier. Based on the preliminary studies, optimal compositions of microparticles, tablets and lyophilisates were chosen. Obtained dosage forms were characterized for drug content, disintegration time and mechanical properties. In order to determine whether the microparticles subjected to direct compression and freeze-drying process effectively mask the bitter taste of cetirizine dihydrochloride, the in vivo and in vitro evaluation was performed. The results showed that designed formulates with microparticles containing cetirizine dihydrochloride were characterized by appropriate mechanical properties, uniformity of weight and thickness, short disintegration time, and the uniform content of the drug substance. Taste-masking assessment performed by three independent methods (e-tongue evaluation, human test panel and the in vitro drug release) revealed that microparticles with Eudragit® E PO are effective taste - masking carriers of cetirizine dihydrochloride and might be used to formulate orally disintegrating tablets and oral lyophilisates.
Keywords: Cetirizine dihydrochloride; E-tongue; Oral lyophilisates; Orally disintegrating tablets; Taste masking.
Figures


Similar articles
-
Original research paper. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying.Acta Pharm. 2017 Mar 1;67(1):113-124. doi: 10.1515/acph-2017-0002. Acta Pharm. 2017. PMID: 28231047
-
In-vitro and in-vivo evaluation of taste-masked cetirizine hydrochloride formulated in oral lyophilisates.Int J Pharm. 2015 Aug 1;491(1-2):8-16. doi: 10.1016/j.ijpharm.2015.06.002. Epub 2015 Jun 5. Int J Pharm. 2015. PMID: 26051543 Clinical Trial.
-
Orodispersible films and tablets with prednisolone microparticles.Eur J Pharm Sci. 2015 Jul 30;75:81-90. doi: 10.1016/j.ejps.2015.04.006. Epub 2015 Apr 16. Eur J Pharm Sci. 2015. PMID: 25889975
-
Orally disintegrating dosage forms and taste-masking technologies; 2010.Expert Opin Drug Deliv. 2011 May;8(5):665-75. doi: 10.1517/17425247.2011.566553. Epub 2011 Mar 27. Expert Opin Drug Deliv. 2011. PMID: 21438776 Review.
-
Orally disintegrating systems: innovations in formulation and technology.Recent Pat Drug Deliv Formul. 2008;2(3):258-74. doi: 10.2174/187221108786241660. Recent Pat Drug Deliv Formul. 2008. PMID: 19075912 Review.
Cited by
-
Taste Masking of Primaquine Phosphate: A Comparative Evaluation of Three Taste Masking Agents.AAPS PharmSciTech. 2025 Jun 11;26(5):168. doi: 10.1208/s12249-025-03162-z. AAPS PharmSciTech. 2025. PMID: 40500474
-
Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate.Polymers (Basel). 2019 Mar 20;11(3):522. doi: 10.3390/polym11030522. Polymers (Basel). 2019. PMID: 30960506 Free PMC article.
-
Critical View on the Qualification of Electronic Tongues Regarding Their Performance in the Development of Peroral Drug Formulations with Bitter Ingredients.Pharmaceutics. 2024 May 15;16(5):658. doi: 10.3390/pharmaceutics16050658. Pharmaceutics. 2024. PMID: 38794320 Free PMC article. Review.
-
Exploitation of Design-of-Experiment Approach for Design and Optimization of Fast-Disintegrating Tablets for Sublingual Delivery of Sildenafil Citrate with Enhanced Bioavailability Using Fluid-Bed Granulation Technique.Pharmaceutics. 2021 Jun 12;13(6):870. doi: 10.3390/pharmaceutics13060870. Pharmaceutics. 2021. PMID: 34204781 Free PMC article.
-
Taste Sensor Assessment of Bitterness in Medicines: Overview and Recent Topics.Sensors (Basel). 2024 Jul 24;24(15):4799. doi: 10.3390/s24154799. Sensors (Basel). 2024. PMID: 39123846 Free PMC article. Review.
References
-
- Ahn S.R., An J.H., Song H.S., Park J.W., Lee S.H., Kim J.H., Jang J., Park T.H. Duplex bioelectronic tongue for sensing umami and sweet tastes based on human taste receptor nanovesicles. ACS Nano. 2016;8:7287–7296. - PubMed
-
- Al-khattawi A., Mohammed A.R. Compressed orally disintegrating tablets: excipients evolution and formulation strategies. Expert Opin. Drug Deliv. 2013;5:651–663. - PubMed
-
- Amelian A., Szekalska M., Ciosek P., Basa A., Winnicka K. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying. Acta Pharm. 2017;67 - PubMed
-
- Amelian A., Winnicka K. Effect of the type of disintegrant on the characteristics of orally disintegrating tablets manufactured using new ready-to-use excipients (Ludiflash® or Parteck®) by direct compression method. African J. Pharmacy Pharmacol. 2012;31:2359–2367.
-
- Badgujar B.P., Mundada A.S. The technologies used for developing orally disintegrating tablets: a review. Acta Pharm. 2011;2:117–139. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

