Effect of neat and binary vehicle systems on the solubility and cutaneous delivery of piperine
- PMID: 30166912
- PMCID: PMC6111231
- DOI: 10.1016/j.jsps.2017.12.015
Effect of neat and binary vehicle systems on the solubility and cutaneous delivery of piperine
Abstract
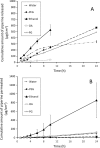
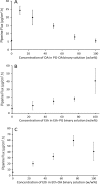
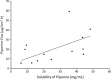
Vitiligo is a skin disease characterized by depigmentation disorders due to lack of melanin production. Piperine, an alkaloid extracted from black piper, is active in melanocytes proliferation. To achieve this, the drug has to reach the melanocytes which exist in the deep layer of the epidermis. Higher drug concentration can be obtained after application of optimized formulation to skin. Accordingly, the aim of this work is to investigate the effect of vehicles on skin penetration of piperine as the first step in development of optimized formulation. The tested vehicles include ethanol (Eth), propylene glycol (PG), polyethylene glycol 400 (PEG), and oleic acid (OA) and their combinations. Water was used as the control and skin permeation was monitored using rabbit ear model skin. The highest piperine solubility (48.6 mg/ml) and flux (40.8 μg/cm2 h) was achieved by Eth and the lowest piperine flux (1.17 μg/cm2 h) was reported for PEG. PG and OA showed piperine flux values comparable to that of the control. Among different combination systems, Eth-OA (75:25) binary system had the highest piperine flux (59.3 μg/cm2 h) followed by Eth-OA (50:50) (32.3 μg/cm2 h) and PG-OA (90:10) (22.7 μg/cm2 h). The study thus introduced a vehicle system as the first step in the development of topical formulation of piperine.
Keywords: Binary system; Cutaneous delivery; Piperine; Vitiligo.
Figures
Similar articles
-
Transdermal delivery of nicardipine: an approach to in vitro permeation enhancement.Drug Deliv. 2002 Oct-Dec;9(4):239-47. doi: 10.1080/10717540260397855. Drug Deliv. 2002. PMID: 12511202
-
In vitro permeation of carvedilol through porcine skin: effect of vehicles and penetration enhancers.PDA J Pharm Sci Technol. 2008 Jul-Aug;62(4):256-63. PDA J Pharm Sci Technol. 2008. PMID: 19174954
-
Transdermal delivery of tadalafil. I. Effect of vehicles on skin permeation.Drug Dev Ind Pharm. 2009 Mar;35(3):329-36. doi: 10.1080/03639040802360494. Drug Dev Ind Pharm. 2009. PMID: 18821150
-
Transdermal delivery of zidovudine: effect of vehicles on permeation across rat skin and their mechanism of action.Eur J Pharm Sci. 2003 Jan;18(1):71-9. doi: 10.1016/s0928-0987(02)00242-7. Eur J Pharm Sci. 2003. PMID: 12554075
-
Microemulsions as transdermal drug delivery vehicles.Adv Colloid Interface Sci. 2006 Nov 16;123-126:369-85. doi: 10.1016/j.cis.2006.05.014. Epub 2006 Jul 14. Adv Colloid Interface Sci. 2006. PMID: 16843424 Review.
Cited by
-
Mucosal Delivery of Cannabidiol: Influence of Vehicles and Enhancers.Pharmaceutics. 2022 Aug 13;14(8):1687. doi: 10.3390/pharmaceutics14081687. Pharmaceutics. 2022. PMID: 36015313 Free PMC article.
-
Vitiligo: From mechanisms of disease to treatable pathways.Skin Health Dis. 2024 Sep 30;4(6):e460. doi: 10.1002/ski2.460. eCollection 2024 Dec. Skin Health Dis. 2024. PMID: 39624766 Free PMC article. Review.
References
-
- Badran M., Alhazza F.I., Alomrani A.H. Development of piperine loaded deformable liposomes - a new vesicular carrier of piperine: characterization and ex vivo skin penetration studies. Lat. Am. J. Pharm. 2015;34:244–252.
-
- Bajad S., Johri R.K., Singh K., Singh J., Bedi K.L. Simple high-performance liquid chromatography method for the simultaneous determination of ketoconazole and piperine in rat plasma and hepatocyte culture. J. Chromatogr. A. 2002;949:43–47. - PubMed
-
- Faas L., Venkatasamy R.C., Hider A.R., Young S.A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008;158:941–950. - PubMed
-
- Falabella R., Barona M.I. Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res. 2008;22:42–65. - PubMed
-
- Fang J.Y., Fang C.L., Liu C.H., Su Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2008;70:633–640. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

