From electron crystallography of 2D crystals to MicroED of 3D crystals
- PMID: 30166936
- PMCID: PMC6112780
- DOI: 10.1016/j.cocis.2018.01.010
From electron crystallography of 2D crystals to MicroED of 3D crystals
Abstract
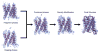
Electron crystallography is widespread in material science applications, but for biological samples its use has been restricted to a handful of examples where two-dimensional (2D) crystals or helical samples were studied either by electron diffraction and/or imaging. Electron crystallography in cryoEM, was developed in the mid-1970s and used to solve the structure of several membrane proteins and some soluble proteins. In 2013, a new method for cryoEM was unveiled and named Micro-crystal Electron Diffraction, or MicroED, which is essentially three-dimensional (3D) electron crystallography of microscopic crystals. This method uses truly 3D crystals, that are about a billion times smaller than those typically used for X-ray crystallography, for electron diffraction studies. There are several important differences and some similarities between electron crystallography of 2D crystals and MicroED. In this review, we describe the development of these techniques, their similarities and differences, and offer our opinion of future directions in both fields.
Keywords: Electron diffraction; MicroED; cryoEM (electorn cryo-microscopy); electron crystallography.
Figures
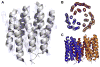




Similar articles
-
In situ protein micro-crystal fabrication by cryo-FIB for electron diffraction.Biophys Rep. 2018;4(6):339-347. doi: 10.1007/s41048-018-0075-x. Epub 2018 Nov 14. Biophys Rep. 2018. PMID: 30596142 Free PMC article.
-
MicroED opens a new era for biological structure determination.Curr Opin Struct Biol. 2016 Oct;40:128-135. doi: 10.1016/j.sbi.2016.09.007. Epub 2016 Oct 1. Curr Opin Struct Biol. 2016. PMID: 27701014 Free PMC article. Review.
-
MicroED: a versatile cryoEM method for structure determination.Emerg Top Life Sci. 2018 Apr 20;2(1):1-8. doi: 10.1042/ETLS20170082. Epub 2018 Feb 6. Emerg Top Life Sci. 2018. PMID: 30167465 Free PMC article.
-
Advances and applications of microcrystal electron diffraction (MicroED).Curr Opin Struct Biol. 2024 Feb;84:102741. doi: 10.1016/j.sbi.2023.102741. Epub 2023 Dec 11. Curr Opin Struct Biol. 2024. PMID: 38086321 Free PMC article. Review.
-
Studying Membrane Protein Structures by MicroED.Methods Mol Biol. 2021;2302:137-151. doi: 10.1007/978-1-0716-1394-8_8. Methods Mol Biol. 2021. PMID: 33877626 Free PMC article.
Cited by
-
The CryoEM Method MicroED as a Powerful Tool for Small Molecule Structure Determination.ACS Cent Sci. 2018 Nov 28;4(11):1587-1592. doi: 10.1021/acscentsci.8b00760. Epub 2018 Nov 2. ACS Cent Sci. 2018. PMID: 30555912 Free PMC article.
-
Macromolecular Nanocrystal Structural Analysis with Electron and X-Rays: A Comparative Review.Molecules. 2019 Sep 26;24(19):3490. doi: 10.3390/molecules24193490. Molecules. 2019. PMID: 31561479 Free PMC article. Review.
-
Collection of Continuous Rotation MicroED Data from Ion Beam-Milled Crystals of Any Size.Structure. 2019 Mar 5;27(3):545-548.e2. doi: 10.1016/j.str.2018.12.003. Epub 2019 Jan 17. Structure. 2019. PMID: 30661853 Free PMC article.
-
In situ protein micro-crystal fabrication by cryo-FIB for electron diffraction.Biophys Rep. 2018;4(6):339-347. doi: 10.1007/s41048-018-0075-x. Epub 2018 Nov 14. Biophys Rep. 2018. PMID: 30596142 Free PMC article.
-
Qualitative Analyses of Polishing and Precoating FIB Milled Crystals for MicroED.Structure. 2019 Oct 1;27(10):1594-1600.e2. doi: 10.1016/j.str.2019.07.004. Epub 2019 Aug 15. Structure. 2019. PMID: 31422911 Free PMC article.
References
-
- Doerr A. Cryo-electron tomography. Nat Methods. 2017;14:34. doi: 10.1038/nmeth.4115. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
