A novel fatty acid-binding protein 5-estrogen-related receptor α signaling pathway promotes cell growth and energy metabolism in prostate cancer cells
- PMID: 30167092
- PMCID: PMC6114981
- DOI: 10.18632/oncotarget.25878
A novel fatty acid-binding protein 5-estrogen-related receptor α signaling pathway promotes cell growth and energy metabolism in prostate cancer cells
Abstract
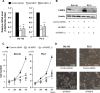
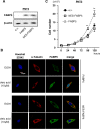
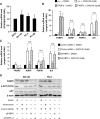
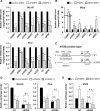
Epidermal or cutaneous fatty acid-binding protein is an intracellular lipid-binding protein, also known as FABP5, and its expression level is closely related to cancer cell proliferation and metastatic activities in various types of carcinoma. However, the molecular mechanisms of FABP5 in cancer cell proliferation and its other functions have remained unclear. In the present study, we have clearly revealed that FABP5 activated expression of metabolic genes (ATP5B, LCHAD, ACO2, FH and MFN2) via a novel signaling pathway in an ERRα (estrogen-related receptor α)-dependent manner in prostate cancer cell lines. To clarify the novel function of FABP5, we examined the activation mechanisms of the ERRα target genes via FABP5. A direct protein-protein interaction between FABP5 and ERRα was demonstrated by immunoprecipitation and GST pull-down assays. We have clearly revealed that FABP5 interacted directly with transcriptional complex containing ERRα and its co-activator PGC-1β to increase expression of the ERRα target genes. In addition, we have shown that FABP5 knockdown induced high energy stress leading to induction of apoptosis and cell cycle arrest via AMPK-FOXO3A signaling pathway in prostate cancer cells, suggesting that FABP5 plays an important role in cellular energy status directing metabolic adaptation to support cellular proliferation and survival.
Keywords: ERRα; FABP5; PGC-1β; energy metabolism; prostate cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures
Similar articles
-
Fatty acid-binding protein 5 (FABP5) promotes lipolysis of lipid droplets, de novo fatty acid (FA) synthesis and activation of nuclear factor-kappa B (NF-κB) signaling in cancer cells.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Sep;1863(9):1057-1067. doi: 10.1016/j.bbalip.2018.06.010. Epub 2018 Jun 12. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29906613
-
Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle.Mol Cell Biol. 2004 Oct;24(20):9079-91. doi: 10.1128/MCB.24.20.9079-9091.2004. Mol Cell Biol. 2004. PMID: 15456881 Free PMC article.
-
ERRα augments HIF-1 signalling by directly interacting with HIF-1α in normoxic and hypoxic prostate cancer cells.J Pathol. 2014 May;233(1):61-73. doi: 10.1002/path.4329. Epub 2014 Feb 5. J Pathol. 2014. PMID: 24425001
-
Cholesterol as an Endogenous ERRα Agonist: A New Perspective to Cancer Treatment.Front Endocrinol (Lausanne). 2018 Sep 11;9:525. doi: 10.3389/fendo.2018.00525. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30254608 Free PMC article. Review.
-
The emerging role of fatty acid binding protein 5 (FABP5) in cancers.Drug Discov Today. 2023 Jul;28(7):103628. doi: 10.1016/j.drudis.2023.103628. Epub 2023 May 23. Drug Discov Today. 2023. PMID: 37230284 Review.
Cited by
-
Lipid metabolism reprogramming in renal cell carcinoma.Cancer Metastasis Rev. 2022 Mar;41(1):17-31. doi: 10.1007/s10555-021-09996-w. Epub 2021 Nov 6. Cancer Metastasis Rev. 2022. PMID: 34741716 Free PMC article. Review.
-
Transcriptomic analysis of the mouse retina after acute and chronic normobaric and hypobaric hypoxia.Sci Rep. 2021 Aug 17;11(1):16666. doi: 10.1038/s41598-021-96150-9. Sci Rep. 2021. PMID: 34404875 Free PMC article.
-
An Amplified Fatty Acid-Binding Protein Gene Cluster in Prostate Cancer: Emerging Roles in Lipid Metabolism and Metastasis.Cancers (Basel). 2020 Dec 18;12(12):3823. doi: 10.3390/cancers12123823. Cancers (Basel). 2020. PMID: 33352874 Free PMC article. Review.
-
FABP5 as a novel molecular target in prostate cancer.Drug Discov Today. 2020 Sep 20:S1359-6446(20)30375-5. doi: 10.1016/j.drudis.2020.09.018. Online ahead of print. Drug Discov Today. 2020. PMID: 32966866 Free PMC article. Review.
-
Fatty-Acid-Binding Proteins: From Lipid Transporters to Disease Biomarkers.Biomolecules. 2023 Dec 6;13(12):1753. doi: 10.3390/biom13121753. Biomolecules. 2023. PMID: 38136624 Free PMC article. Review.
References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–28. - PubMed
-
- Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

