Preparation of Precisely Oriented Cryosections of Undistorted Drosophila Wing Imaginal Discs for High Resolution Confocal Imaging
- PMID: 30167436
- PMCID: PMC6112823
- DOI: 10.21769/BioProtoc.2725
Preparation of Precisely Oriented Cryosections of Undistorted Drosophila Wing Imaginal Discs for High Resolution Confocal Imaging
Abstract
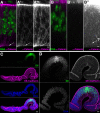
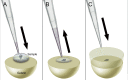
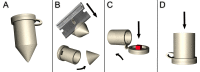
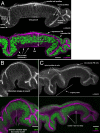
The combination of immunofluorescence and laser scanning confocal microscopy (LSM) is essential to high-resolution detection of molecular distribution in biological specimens. A frequent limitation is the need to image deep inside a tissue or in a specific plane, which may be inaccessible due to tissue size or shape. Recreating high-resolution 3D images is not possible because the point-spread function of light reduces the resolution in the Z-axis about 3-fold, compared to XY, and light scattering obscures signal deep in the tissue. However, the XY plane of interest can be chosen if embedded samples are precisely oriented and sectioned prior to imaging (Figure 1). Here we describe the preparation of frozen tissue sections of the Drosophila wing imaginal disc, which allows us to obtain high-resolution images throughout the depth of this folded epithelium.
Keywords: Confocal microscopy; Cryosection; Drosophila; Frozen sections; Wing imaginal disc.
Figures



Similar articles
-
Histological analysis of the dynamics of growth of imaginal discs and histoblast nests during the larval development ofDrosophila melanogaster.Wilehm Roux Arch Dev Biol. 1977 Dec;183(4):269-305. doi: 10.1007/BF00848459. Wilehm Roux Arch Dev Biol. 1977. PMID: 28304865
-
Large-scale imaginal disc sorting: A protocol for "omics"-approaches.Methods. 2014 Jun 15;68(1):260-4. doi: 10.1016/j.ymeth.2014.04.005. Epub 2014 Apr 13. Methods. 2014. PMID: 24736056 Review.
-
Towards long term cultivation of Drosophila wing imaginal discs in vitro.PLoS One. 2014 Sep 9;9(9):e107333. doi: 10.1371/journal.pone.0107333. eCollection 2014. PLoS One. 2014. PMID: 25203426 Free PMC article.
-
Induction and Diagnosis of Tumors in Drosophila Imaginal Disc Epithelia.J Vis Exp. 2017 Jul 25;(125):55901. doi: 10.3791/55901. J Vis Exp. 2017. PMID: 28784954 Free PMC article.
-
Thin is better!: ultrathin cryosection immunocytochemistry.J Nippon Med Sch. 2004 Oct;71(5):306-7. doi: 10.1272/jnms.71.306. J Nippon Med Sch. 2004. PMID: 15514446 Review.
Cited by
-
Destruction complex dynamics: Wnt/β-catenin signaling alters Axin-GSK3β interactions in vivo.Development. 2019 Jul 2;146(13):dev164145. doi: 10.1242/dev.164145. Development. 2019. PMID: 31189665 Free PMC article.
References
-
- Cohen S. M.(1993). Imaginal disc development. In: Bate, M. and Martinez Arias, A.(Eds.). The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; pp: 747-841.
-
- Klein T.(2008). Immunolabeling of imaginal discs. In: Dahmann, C.(Ed.). Drosophila: Methods and Protocols . Methods Mol Biol 420: 253-263. - PubMed
-
- McClure K. D. and Schubiger G.(2005). Developmental analysis and squamous morphogenesis of the peripodial epithelium in Drosophila imaginal discs . Development 132(22): 5033-5042. - PubMed
-
- Sui L., Pflugfelder G. O. and Shen J.(2012). The Dorsocross T-box transcription factors promote tissue morphogenesis in the Drosophila wing imaginal disc . Development 139(15): 2773-2782. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

