Electrophoretic drug delivery for seizure control
- PMID: 30167463
- PMCID: PMC6114990
- DOI: 10.1126/sciadv.aau1291
Electrophoretic drug delivery for seizure control
Abstract
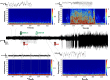
The persistence of intractable neurological disorders necessitates novel therapeutic solutions. We demonstrate the utility of direct in situ electrophoretic drug delivery to treat neurological disorders. We present a neural probe incorporating a microfluidic ion pump (μFIP) for on-demand drug delivery and electrodes for recording local neural activity. The μFIP works by electrophoretically pumping ions across an ion exchange membrane and thereby delivers only the drug of interest and not the solvent. This "dry" delivery enables precise drug release into the brain region with negligible local pressure increase. The therapeutic potential of the μFIP probe is tested in a rodent model of epilepsy. The μFIP probe can detect pathological activity and then intervene to stop seizures by delivering inhibitory neurotransmitters directly to the seizure source. We anticipate that further tailored engineering of the μFIP platform will enable additional applications in neural interfacing and the treatment of neurological disorders.
Figures




References
-
- Dong S., Rogan S. C., Roth B. L., Directed molecular evolution of DREADDs: A generic approach to creating next-generation RASSLs. Nat. Protoc. 5, 561–573 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

