Needs-Based Innovation in Cardiovascular Medicine: The Stanford Biodesign Process
- PMID: 30167537
- PMCID: PMC6113348
- DOI: 10.1016/j.jacbts.2016.06.011
Needs-Based Innovation in Cardiovascular Medicine: The Stanford Biodesign Process
Abstract
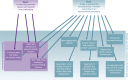
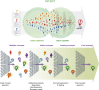
More than a decade ago, a formalized fellowship training program in medical device innovation, the first of its kind, was created at Stanford University. Now in its 15th year, the Stanford Biodesign Fellowship Program is a 10-month program whereby postgraduate students with a prior background in medicine, engineering, and/or business form interdisciplinary teams for an experiential process of identifying unmet clinical needs, inventing new solutions, and implementing these ideas (the 3 "I's"). A key component of this structured process is focused attention on needs finding and characterization, which differs from the traditional "tech-push" model (i.e., technologies looking for problems to solve). Although the Stanford Biodesign process can be applied to a wide variety of clinical areas, cardiovascular medicine is particularly well suited, given the breadth of clinical presentations it touches and its history of innovation to solve important clinical problems. Physicians play a vital role in the process, especially for needs identification and characterization. This paper outlines the Stanford Biodesign process and presents an argument for its repeat applicability, discusses its relevance to physicians and to cardiologists in particular, and provides a case study of the process that resulted in a currently available cardiovascular medical technology that came directly from the Fellowship Program.
Keywords: EP, electrophysiologist/electrophysiology; IP, intellectual property; Stanford; biodesign; cardiology; innovation; invention; medical device; medical technology; needs-based; translational.
Figures




References
-
- Wall J., Wynne E., Krummel T. Biodesign process and culture to enable pediatric medical technology innovation. Semin Peadiatr Surg. 2015;24:102–106. - PubMed
-
- Yock P.G., Brinton T.J., Zenios S.A. Teaching biomedical innovation as a discipline. Sci Transl Med. 2011;3:92cm18. - PubMed
-
- Yock P.G., Linker D.T., Angelsen B.A. Two-dimensional intravascular ultrasound: technical development and initial clinical experience. J Am Soc Echocardiogr. 1989;2:296–304. - PubMed
-
- Yock P.G., Zenios S., Makower J. 2nd edition. Cambridge University Press; Cambridge, UK: 2015. Biodesign: The Process of Innovating Medical Technologies.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

