A Phase I, Single Ascending Dose Study of Cimaglermin Alfa (Neuregulin 1β3) in Patients With Systolic Dysfunction and Heart Failure
- PMID: 30167542
- PMCID: PMC6113538
- DOI: 10.1016/j.jacbts.2016.09.005
A Phase I, Single Ascending Dose Study of Cimaglermin Alfa (Neuregulin 1β3) in Patients With Systolic Dysfunction and Heart Failure
Abstract
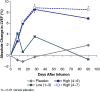
A first-in-human, phase 1, double blind, placebo-controlled, single ascending dose study examined the safety, tolerability, and exploratory efficacy of intravenous infusion of a recombinant growth factor, cimaglermin alfa, in patients with heart failure and left ventricular systolic dysfunction (LVSD). In these patients on optimal guideline-directed medical therapy, cimaglermin treatment was generally tolerated except for transient nausea and headache and a dose-limiting toxicity was noted at the highest planned dose. There was a dose-dependent improvement in left ventricular ejection fraction lasting 90 days following infusion. Thus, cimaglermin is a potential therapy to enhance cardiac function in LVSD and warrants further investigation.
Keywords: AE, adverse event; AUC, area under the curve; DLT, dose-limiting toxicity; GGF, glial growth factor; HF, heart failure; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; NRG, neuregulin; NYHA, New York Heart Association functional class; TEAE, treatment-emergent adverse event; cardiac repair; growth factor; neuregulin; systolic dysfunction.
Figures


References
-
- Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1:1–20. - PubMed
-
- Cubbon R.M., Gale C.P., Kearney L.C. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. - PubMed
-
- McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. - PubMed
-
- Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

