Opioid Prescribing in the United States Before and After the Centers for Disease Control and Prevention's 2016 Opioid Guideline
- PMID: 30167651
- PMCID: PMC6176709
- DOI: 10.7326/M18-1243
Opioid Prescribing in the United States Before and After the Centers for Disease Control and Prevention's 2016 Opioid Guideline
Abstract
Background: In response to adverse outcomes from prescription opioids, the Centers for Disease Control and Prevention (CDC) released the Guideline for Prescribing Opioids for Chronic Pain in March 2016.
Objective: To test the hypothesis that the CDC guideline release corresponded to declines in specific opioid prescribing practices.
Design: Interrupted time series analysis of monthly prescribing measures from the IQVIA transactional data warehouse and Real-World Data Longitudinal Prescriptions population-level estimates based on retail pharmacy data. Population size was determined by U.S. Census monthly estimates.
Setting: United States, 2012 to 2017.
Patients: Persons prescribed opioid analgesics.
Measurements: Outcomes included opioid dosage, days supplied, overlapping benzodiazepine prescriptions, and the overall rate of prescribing.
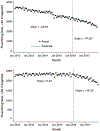
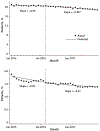
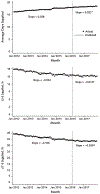
Results: The rate of high-dosage prescriptions (≥90 morphine equivalent milligrams per day) was 683 per 100 000 persons in January 2012 and declined by 3.56 (95% CI, -3.79 to -3.32) per month before March 2016 and by 8.00 (CI, -8.69 to -7.31) afterward. Likewise, the percentage of patients with overlapping opioid and benzodiazepine prescriptions was 21.04% in January 2012 and declined by 0.02% (CI, -0.04% to -0.01%) per month before the CDC guideline release and by 0.08% (CI, -0.08% to -0.07%) per month afterward. The overall opioid prescribing rate was 6577 per 100 000 persons in January 2012 and declined by 23.48 (CI, -26.18 to -20.78) each month before the guideline release and by 56.74 (CI, -65.96 to -47.53) per month afterward.
Limitation: No control population; inability to determine the appropriateness of opioid prescribing.
Conclusion: Several opioid prescribing practices were decreasing before the CDC guideline, but the time of its release was associated with a greater decline. Guidelines may be effective in changing prescribing practices.
Primary funding source: CDC.
Conflict of interest statement
Figures


Comment in
-
Opioid Prescribing Before and After the Centers for Disease Control and Prevention's 2016 Opioid Guideline.Ann Intern Med. 2019 Apr 16;170(8):581-582. doi: 10.7326/L19-0053. Ann Intern Med. 2019. PMID: 30986838 No abstract available.
References
-
- Hedegaard H, Warner M, Miniño AM Drug overdose deaths in the United States, 1999–2016 National Center for Health Statistics data brief. Hyattsville, MD: National Center for Health Statistics; 2017.
-
- Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59:705–9. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
