Inflammaging impairs peripheral nerve maintenance and regeneration
- PMID: 30168637
- PMCID: PMC6260910
- DOI: 10.1111/acel.12833
Inflammaging impairs peripheral nerve maintenance and regeneration
Abstract
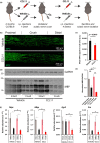
The regenerative capacity of peripheral nerves declines during aging, contributing to the development of neuropathies, limiting organism function. Changes in Schwann cells prompt failures in instructing maintenance and regeneration of aging nerves; molecular mechanisms of which have yet to be delineated. Here, we identified an altered inflammatory environment leading to a defective Schwann cell response, as an underlying mechanism of impaired nerve regeneration during aging. Chronic inflammation was detected in intact uninjured old nerves, characterized by increased macrophage infiltration and raised levels of monocyte chemoattractant protein 1 (MCP1) and CC chemokine ligand 11 (CCL11). Schwann cells in the old nerves appeared partially dedifferentiated, accompanied by an activated repair program independent of injury. Upon sciatic nerve injury, an initial delayed immune response was followed by a persistent hyperinflammatory state accompanied by a diminished repair process. As a contributing factor to nerve aging, we showed that CCL11 interfered with Schwann cell differentiation in vitro and in vivo. Our results indicate that increased infiltration of macrophages and inflammatory signals diminish regenerative capacity of aging nerves by altering Schwann cell behavior. The study identifies CCL11 as a promising target for anti-inflammatory therapies aiming to improve nerve regeneration in old age.
Keywords: aging; inflammaging; macrophages; neural regeneration; peripheral nervous system; schwann cell.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures






References
-
- Boivin, A. , Pineau, I. , Barrette, B. , Filali, M. , Vallieres, N. , Rivest, S. , & Lacroix, S. (2007). Toll‐like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 27, 12565–12576. 10.1523/JNEUROSCI.3027-07.2007 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

