Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels
- PMID: 30169422
- PMCID: PMC6309937
- DOI: 10.1097/j.pain.0000000000001385
Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels
Abstract
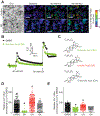
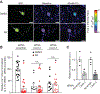
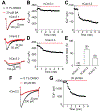
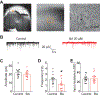
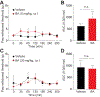
The Federal Pain Research Strategy recommended development of nonopioid analgesics as a top priority in its strategic plan to address the significant public health crisis and individual burden of chronic pain faced by >100 million Americans. Motivated by this challenge, a natural product extracts library was screened and identified a plant extract that targets activity of voltage-gated calcium channels. This profile is of interest as a potential treatment for neuropathic pain. The active extract derived from the desert lavender plant native to southwestern United States, when subjected to bioassay-guided fractionation, afforded 3 compounds identified as pentacyclic triterpenoids, betulinic acid (BA), oleanolic acid, and ursolic acid. Betulinic acid inhibited depolarization-evoked calcium influx in dorsal root ganglion (DRG) neurons predominantly through targeting low-voltage-gated (Cav3 or T-type) and CaV2.2 (N-type) calcium channels. Voltage-clamp electrophysiology experiments revealed a reduction of Ca, but not Na, currents in sensory neurons after BA exposure. Betulinic acid inhibited spontaneous excitatory postsynaptic currents and depolarization-evoked release of calcitonin gene-related peptide from lumbar spinal cord slices. Notably, BA did not engage human mu, delta, or kappa opioid receptors. Intrathecal administration of BA reversed mechanical allodynia in rat models of chemotherapy-induced peripheral neuropathy and HIV-associated peripheral sensory neuropathy as well as a mouse model of partial sciatic nerve ligation without effects on locomotion. The broad-spectrum biological and medicinal properties reported, including anti-HIV and anticancer activities of BA and its derivatives, position this plant-derived small molecule natural product as a potential nonopioid therapy for management of chronic pain.
Conflict of interest statement
Figures

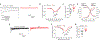


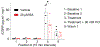

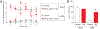
References
-
- Barsa JA, Sauders JC. Amitriptyline (Elavil), a new antidepressant. Am J Psychiatry 1961;117:739–740. - PubMed
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain physician 2008;11(2 Suppl):S105–120. - PubMed
-
- Bildziukevich U, Rarova L, Saman D, Wimmer Z. Picolyl amides of betulinic acid as antitumor agents causing tumor cell apoptosis. European journal of medicinal chemistry 2018;145:41–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

