Binding site matching in rational drug design: algorithms and applications
- PMID: 30169563
- PMCID: PMC6954434
- DOI: 10.1093/bib/bby078
Binding site matching in rational drug design: algorithms and applications
Abstract
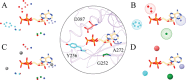
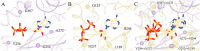
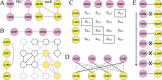
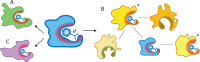
Interactions between proteins and small molecules are critical for biological functions. These interactions often occur in small cavities within protein structures, known as ligand-binding pockets. Understanding the physicochemical qualities of binding pockets is essential to improve not only our basic knowledge of biological systems, but also drug development procedures. In order to quantify similarities among pockets in terms of their geometries and chemical properties, either bound ligands can be compared to one another or binding sites can be matched directly. Both perspectives routinely take advantage of computational methods including various techniques to represent and compare small molecules as well as local protein structures. In this review, we survey 12 tools widely used to match pockets. These methods are divided into five categories based on the algorithm implemented to construct binding-site alignments. In addition to the comprehensive analysis of their algorithms, test sets and the performance of each method are described. We also discuss general pharmacological applications of computational pocket matching in drug repurposing, polypharmacology and side effects. Reflecting on the importance of these techniques in drug discovery, in the end, we elaborate on the development of more accurate meta-predictors, the incorporation of protein flexibility and the integration of powerful artificial intelligence technologies such as deep learning.
Keywords: drug repositioning; drug side effects; off-targets; pocket alignment; pocket matching; polypharmacology.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Geerts H, Hofmann-Apitius M, Anastasio TJ, et al. Knowledge-driven computational modeling in Alzheimer's disease research: current state and future trends. Alzheimers Dement 2017;13:1292–302. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

