Nonoptimal Gene Expression Creates Latent Potential for Antibiotic Resistance
- PMID: 30169679
- PMCID: PMC6231494
- DOI: 10.1093/molbev/msy163
Nonoptimal Gene Expression Creates Latent Potential for Antibiotic Resistance
Abstract
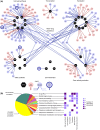
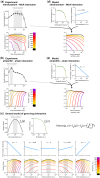
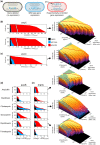
Bacteria regulate genes to survive antibiotic stress, but regulation can be far from perfect. When regulation is not optimal, mutations that change gene expression can contribute to antibiotic resistance. It is not systematically understood to what extent natural gene regulation is or is not optimal for distinct antibiotics, and how changes in expression of specific genes quantitatively affect antibiotic resistance. Here we discover a simple quantitative relation between fitness, gene expression, and antibiotic potency, which rationalizes our observation that a multitude of genes and even innate antibiotic defense mechanisms have expression that is critically nonoptimal under antibiotic treatment. First, we developed a pooled-strain drug-diffusion assay and screened Escherichia coli overexpression and knockout libraries, finding that resistance to a range of 31 antibiotics could result from changing expression of a large and functionally diverse set of genes, in a primarily but not exclusively drug-specific manner. Second, by synthetically controlling the expression of single-drug and multidrug resistance genes, we observed that their fitness-expression functions changed dramatically under antibiotic treatment in accordance with a log-sensitivity relation. Thus, because many genes are nonoptimally expressed under antibiotic treatment, many regulatory mutations can contribute to resistance by altering expression and by activating latent defenses.
Figures


References
-
- Alekshun MN, Levy SB.. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7(10): 410–413. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.. 1990. Basic local alignment search tool. J Mol Biol. 215(3): 403–410. - PubMed
-
- Andersson DI, Hughes D.. 2009. Gene amplification and adaptive evolution in bacteria. Annu Rev Genet. 43:167–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

