Arsenic Alters Exosome Quantity and Cargo to Mediate Stem Cell Recruitment Into a Cancer Stem Cell-Like Phenotype
- PMID: 30169766
- PMCID: PMC6111788
- DOI: 10.1093/toxsci/kfy176
Arsenic Alters Exosome Quantity and Cargo to Mediate Stem Cell Recruitment Into a Cancer Stem Cell-Like Phenotype
Abstract
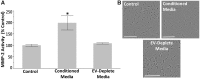
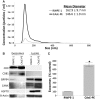
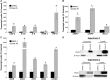
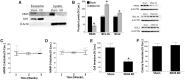
Inorganic arsenic is a human carcinogen that can target the prostate. Accumulating evidence suggests arsenic can disrupt stem cell (SC) dynamics during the carcinogenic process. Previous work demonstrated arsenic-transformed prostate epithelial (CAsE-PE) cells can recruit prostate SCs into rapidly acquiring a cancer SC (CSC) phenotype via the secretion of soluble factors. Exosomes are small, membrane-derived vesicles that contain lipids, RNA, and proteins, and actively contribute to cancer initiation and progression when taken up by target cells. Here we hypothesized that CAsE-PE cells are recruiting SCs to a CSC-like phenotype via exosomal signaling. CAsE-PE cells secreted 700% more exosomes than parental RWPE-1 cells. CAsE-PE exosomes were enriched with oncogenic factors, including oncogenes (KRAS, NRAS, VEFGA, MYB, and EGFR), inflammation-related (cyclooxygenase-2, interleukin 1B (IL1B), IL6, transforming growth factor-β, and tumor necrosis factor-A), and apoptosis-related (CASP7, CASP9, and BCL2) transcripts, and oncogenesis-associated microRNAs. When compared with SCs cultured in exosome-depleted conditioned medium (CM), SCs cultured in CM containing CAsE-PE-derived exosomes showed increased (198%) matrix metalloproteinase activity and underwent an epithelial-to-mesenchymal transition in morphology, suggesting an exosome-mediated transformation. KRAS plays an important role in arsenic carcinogenesis. Although KRAS transcript (>24 000%) and protein (866%) levels were elevated in CAsE-PE exosomes, knock-down of KRAS in these cells only partially mitigated the CSC-like phenotype in cocultured SCs. Collectively, these results suggest arsenic impacts both exosomal quantity and cargo. Exosomal KRAS is only minimally involved in this recruitment, and additional factors (eg, cancer-associated miRNAs) likely also play a role. This work furthers our mechanistic understanding of how arsenic disrupts SC dynamics and influences the tumor microenvironment during carcinogenesis.
Figures
References
-
- Achanzar W. E., Brambila E. M., Diwan B. A., Webber M. M., Waalkes M. P. (2002). Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl Cancer Inst. 94, 1888–1891. - PubMed
-
- Adams R. H., Alitalo K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464.. - PubMed
-
- Bachmeier B., Boukamp P., Lichtinghagen R., Fusenig N., Fink E. (2000). Matrix metalloproteinases-2,-3,-7,-9 and-10, but not MMP-11, are differentially expressed in normal, benign tumorigenic and malignant human keratinocyte cell lines. Biol. Chem. 381, 497–507. - PubMed
-
- Baroni S., Romero-Cordoba S., Plantamura I., Dugo M., D’Ippolito E., Cataldo A., Cosentino G., Angeloni V., Rossini A., Daidone M. G., et al. (2016). Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 7, e2312.. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

