Feminization imprinted by developmental growth hormone
- PMID: 30170181
- PMCID: PMC6263729
- DOI: 10.1016/j.mce.2018.08.011
Feminization imprinted by developmental growth hormone
Abstract
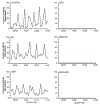
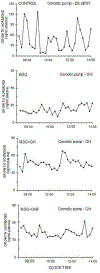
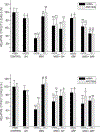
Previously, we identified early developmental exposure to growth hormone (GH) as the requisite organizer responsible for programming the masculinization of the hepatic cytochromes P450 (CYP)-dependent drug metabolizing enzymes (Das et al., 2014, 2017). In spite of the generally held dogma that mammalian feminization requires no hormonal imprinting, numerous reports that the sex-dependent regulation and expression of hepatic CYPs in females are permanent and irreversible would suggest otherwise. Consequently, we selectively blocked GH secretion in a cohort of newborn female rats, some of whom received concurrent GH replacement or GH releasing factor. As adults, the feminine circulating GH profile was restored in the treated animals. Two categories of CYPs were measured. The principal and basically female specific CYP2C12 and CYP2C7; both completely and solely dependent on the adult feminine continuous GH profile for expression, and the female predominant CYP2C6 and CYP2E1 whose expression is maximum in the absence of plasma GH, suppressed by the feminine GH profile but more so by the masculine episodic GH profile. Our findings indicate that early developmental exposure to GH imprints the inchoate CYP2C12 and CYP2C7 in the differentiating liver to be solely dependent on the feminine GH profile for expression in the adult female. In contrast, adult expression of CYP2C6 and CYP2E1 in the female rat appears to require no GH imprinting.
Keywords: Cytochromes P450; Development; Feminization; Growth hormone; Imprinting; Sexual dimorphism.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures
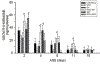



Similar articles
-
Early expression of requisite developmental growth hormone imprinted cytochromes P450 and dependent transcription factors.Endocr Connect. 2021 Sep 20;10(9):1167-1179. doi: 10.1530/EC-21-0143. Endocr Connect. 2021. PMID: 34424855 Free PMC article.
-
Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile.Endocrinology. 1999 Mar;140(3):1245-54. doi: 10.1210/endo.140.3.6545. Endocrinology. 1999. PMID: 10067850
-
Feminization of hepatic cytochrome P450s by nominal levels of growth hormone in the feminine plasma profile.Mol Pharmacol. 1996 Nov;50(5):1148-56. Mol Pharmacol. 1996. PMID: 8913346
-
Role of hepatocyte nuclear factors in growth hormone-regulated, sexually dimorphic expression of liver cytochromes P450.Growth Factors. 2004 Jun;22(2):79-88. doi: 10.1080/08977190410001715172. Growth Factors. 2004. PMID: 15253383 Review.
-
Sexual dimorphism in the control of growth hormone secretion.Endocr Rev. 1985 Spring;6(2):128-50. doi: 10.1210/edrv-6-2-128. Endocr Rev. 1985. PMID: 2861084 Review.
Cited by
-
A spatiotemporal steroidogenic regulatory network in human fetal adrenal glands and gonads.Front Endocrinol (Lausanne). 2022 Nov 17;13:1036517. doi: 10.3389/fendo.2022.1036517. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36465633 Free PMC article.
-
Impact of 3D genome organization, guided by cohesin and CTCF looping, on sex-biased chromatin interactions and gene expression in mouse liver.Epigenetics Chromatin. 2020 Jul 17;13(1):30. doi: 10.1186/s13072-020-00350-y. Epigenetics Chromatin. 2020. PMID: 32680543 Free PMC article.
-
Early expression of requisite developmental growth hormone imprinted cytochromes P450 and dependent transcription factors.Endocr Connect. 2021 Sep 20;10(9):1167-1179. doi: 10.1530/EC-21-0143. Endocr Connect. 2021. PMID: 34424855 Free PMC article.
References
-
- Agrawal AK, Pampori NA & Shapiro BH 1995. Neonatal phenobarbital-induced defects in age- and sex-specific growth hormone profiles regulating monooxygenases. American Journal of Physiology 268 E439–445. - PubMed
-
- Agrawal AK & Shapiro BH 1997. Gender, age and dose effects of neonatally administered aspartate on the sexually dimorphic plasma growth hormone profiles regulating expression of the rat sex-dependent hepatic CYP isoforms. Drug Metabolism and Disposition 25 1249–1256. - PubMed
-
- Agrawal AK & Shapiro BH 2000. Differential expression of gender-dependent hepatic isoforms of cytochrome P-450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. Journal of Pharmacology and Experimental Therapeutics 292 228–237. - PubMed
-
- Ahluwalia A, Clodfelter KH &Waxman DJ 2004. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Molecular Endocrinology 18 747–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

