Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: a case report
- PMID: 30170630
- PMCID: PMC6117974
- DOI: 10.1186/s40425-018-0396-9
Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: a case report
Abstract
Background: Immune checkpoint inhibitors (ICIs) are the treatment of choice for several cancers and can be associated with remarkable clinical benefit, but can also cause serious immune-related adverse events (irAEs). Management of rare and severe irAEs is challenged by an incomplete knowledge of their natural history and pathogenetic mechanisms. We report a case of fatal acute-onset gastro-intestinal (GI) hypomotility from myenteric plexus neuropathy following a single dose of ipilimumab plus nivolumab given for treatment of Merkel cell carcinoma (MCC).
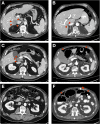
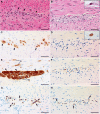
Case presentation: A 66-year-old man with recurrent metastatic MCC involving several organs (liver, bones and disseminated retroperitoneal lymphadenopathy) developed profound pharyngeal dysphagia and ileus that started 7 days after receiving a single administration of combination immune checkpoint blockade consisting of nivolumab (3 mg/kg) and low-dose ipilimumab (1 mg/kg). A swallowing study showed oropharyngeal dysfunction and aspiration. Imaging studies were consistent with diffuse intestinal paresis. An extensive work-up did not reveal obvious causes of these symptoms, and enteric plexopathy was suspected. Empiric immune suppressive therapy was initiated urgently. Despite an escalating immunosuppressive regimen that included high dose steroids, tacrolimus and therapeutic plasma exchange, no improvement in GI motility was seen and the patient suffered repeated episodes of aspiration. Seven weeks after the onset of GI hypomotility, the patient succumbed to sepsis from intestinal perforations. At autopsy, histologic specimens obtained from the entire GI tract (pharynx to rectum) showed near complete loss of ganglion cells within the myenteric and submucosal plexuses. An associated inflammatory infiltrate was not seen, suggesting a 'burned out' phase of illness. C4d complement deposition was found at the ganglionic sites, suggesting antibody-mediated pathogenesis. Remarkably, at sites of previously suspected Merkel cell metastases, no residual viable Merkel cell carcinoma was identified.
Conclusions: GI-tract paresis due to myenteric neuritis is a rarely reported toxicity of ICIs. Because the window of reversibility is likely to be very brief, quick and decisive interventions are warranted. Subtle functional and anatomic perturbations of the myenteric nervous system from the use of ICIs may be more prevalent than realized and should be suspected and addressed in both clinical and investigational settings.
Trial registration: ClinicalTrials.gov NCT02488759.
Keywords: Enteric neuropathy; Ileus; Immune checkpoint inhibitor; Immune-related adverse events; Ipilimumab; Merkel cell; Myenteric plexopathy; Neuritis; Nivolumab; Pseudo-obstruction.
Conflict of interest statement
Ethics approval and consent to participate
The patient had provided informed consent for sharing his information on a research protocol approved by the University of Washington/Fred Hutchison Cancer Research Center IRB.
Consent for publication
The patient and his wife had provided consent for the use of the medical information contained in this case report for research and publication.
Competing interests
SB reports institutional research (to UW) funding from Merck, Bristol Myers Squibb (BMS) and EMD-Serono. SB has participated in advisory boards, receiving honoraria, sponsored by Bristol Myers Squibb (BMS), Genentech and EMD-Serono. PN reports acting as a consultant for EMD Serono, Merck and Pfizer and his laboratory has received research funding from BMS & EMD Serono. The remaining authors declare that they have no competing financial interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
