The Chemokine Receptor CCR8 Promotes the Migration of Dendritic Cells into the Lymph Node Parenchyma to Initiate the Allergic Immune Response
- PMID: 30170811
- PMCID: PMC6192021
- DOI: 10.1016/j.immuni.2018.07.012
The Chemokine Receptor CCR8 Promotes the Migration of Dendritic Cells into the Lymph Node Parenchyma to Initiate the Allergic Immune Response
Abstract
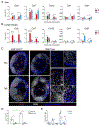
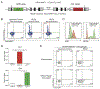
The migration of mature dendritic cells (DCs) into the draining lymph node (dLN) is thought to depend solely on the chemokine receptor CCR7. CD301b+ DCs migrate into the dLN after cutaneous allergen exposure and are required for T helper 2 (Th2) differentiation. We found that CD301b+ DCs poorly upregulated CCR7 expression after allergen exposure and required a second chemokine signal, mediated by CCR8 on CD301b+ DCs and its ligand CCL8, to exit the subcapsular sinus (SCS) and enter the lymph node (LN) parenchyma. After allergen exposure, CD169+SIGN-R1+ macrophages in interfollicular regions produced CCL8, which synergized with CCL21 in a Src-kinase-dependent manner to promote CD301b+ DC migration. In CCR8-deficient mice, CD301b+ DCs remained in the SCS and were unable to enter the LN parenchyma, resulting in defective Th2 differentiation. We have defined a CCR8-dependent stepwise mechanism of DC-subset-specific migration through which LN CD169+SIGN-R1+ macrophages control the polarization of the adaptive immune response.
Keywords: CCL8; CCR8; Th2 differentiation; allergy; chemokines; dendritic cells; lymph node; macrophages; migration; papain.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTEREST
The authors declare no competing interests.
Figures





Comment in
-
Two-Factor Authentication for Type 2 Immunity.Immunity. 2018 Sep 18;49(3):381-383. doi: 10.1016/j.immuni.2018.08.020. Immunity. 2018. PMID: 30231976
References
-
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, and Forster R (2011). Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol 12, 879–887. - PubMed
-
- Chung CD, Kuo F, Kumer J, Motani AS, Lawrence CE, Henderson WR Jr., and Venkataraman C (2003). CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol 170, 581–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

