Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis
- PMID: 30170814
- PMCID: PMC6347470
- DOI: 10.1016/j.immuni.2018.07.006
Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis
Abstract
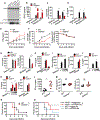
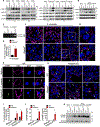
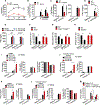
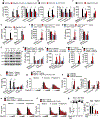
Inflammasome-activated caspase-1 cleaves gasdermin D to unmask its pore-forming activity, the predominant consequence of which is pyroptosis. Here, we report an additional biological role for gasdermin D in limiting cytosolic DNA surveillance. Cytosolic DNA is sensed by Aim2 and cyclic GMP-AMP synthase (cGAS) leading to inflammasome and type I interferon responses, respectively. We found that gasdermin D activated by the Aim2 inflammasome suppressed cGAS-driven type I interferon response to cytosolic DNA and Francisella novicida in macrophages. Similarly, interferon-β (IFN-β) response to F. novicida infection was elevated in gasdermin D-deficient mice. Gasdermin D-mediated negative regulation of IFN-β occurred in a pyroptosis-, interleukin-1 (IL-1)-, and IL-18-independent manner. Mechanistically, gasdermin D depleted intracellular potassium (K+) via membrane pores, and this K+ efflux was necessary and sufficient to inhibit cGAS-dependent IFN-β response. Thus, our findings have uncovered an additional interferon regulatory module involving gasdermin D and K+ efflux.
Keywords: Francisella; IFN-β; K(+); STING; cGAS; caspase-1; cytosolic DNA; gasdermin D; inflammasome; poly(dA:dT); potassium.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Ion Man: GSDMD Punches Pores to Knock Out cGAS.Immunity. 2018 Sep 18;49(3):379-381. doi: 10.1016/j.immuni.2018.08.026. Immunity. 2018. PMID: 30231975
References
-
- Chen Q, Sun L, and Chen ZJ (2016). Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nature Immunology 17, 1142–1149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

