DNA protein crosslink proteolysis repair: From yeast to premature ageing and cancer in humans
- PMID: 30170832
- PMCID: PMC6219452
- DOI: 10.1016/j.dnarep.2018.08.025
DNA protein crosslink proteolysis repair: From yeast to premature ageing and cancer in humans
Abstract
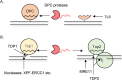
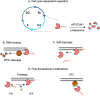
DNA-protein crosslinks (DPCs) are a specific type of DNA lesion consisting of a protein covalently and irreversibly bound to DNA, which arise after exposure to physical and chemical crosslinking agents. DPCs can be bulky and thereby pose a barrier to DNA replication and transcription. The persistence of DPCs during S phase causes DNA replication stress and genome instability. The toxicity of DPCs is exploited in cancer therapy: many common chemotherapeutics kill cancer cells by inducing DPC formation. Recent work from several laboratories discovered a specialized repair pathway for DPCs, namely DPC proteolysis (DPCP) repair. DPCP repair is carried out by replication-coupled DNA-dependent metalloproteases: Wss1 in yeast and SPRTN in metazoans. Mutations in SPRTN cause premature ageing and liver cancer in humans and mice; thus, defective DPC repair has great clinical ramifications. In the present review, we will revise the current knowledge on the mechanisms of DPCP repair and on the regulation of DPC protease activity, while highlighting the most significant unresolved questions in the field. Finally, we will discuss the impact of faulty DPC repair on disease and cancer therapy.
Keywords: Ageing; Cancer; DNA-protein crosslinks; Genome stability; Post-translational modification; SPRTN protease.
Crown Copyright © 2018. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Ide H. 2015. Formation, Repair, and Biological Effects of DNA–Protein Cross-Link Damage.
-
- Szende B., Tyihak E. Effect of formaldehyde on cell proliferation and death. Cell Biol. Int. 2010;34(12):1273–1282. - PubMed
-
- Stingele J., Jentsch S. DNA-protein crosslink repair. Nat. Rev. Mol. Cell Biol. 2015;16(8):455–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

