Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria
- PMID: 30171080
- PMCID: PMC6431239
- DOI: 10.1126/sciimmunol.aat7668
Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria
Abstract
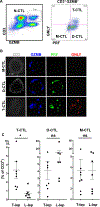
Human CD8+ cytotoxic T lymphocytes (CTLs) contribute to antimicrobial defense against intracellular pathogens through secretion of cytotoxic granule proteins granzyme B, perforin, and granulysin. However, CTLs are heterogeneous in the expression of these proteins, and the subset(s) responsible for antimicrobial activity is unclear. Studying human leprosy, we found that the subset of CTLs coexpressing all three cytotoxic molecules is increased in the resistant form of the disease, can be expanded by interleukin-15 (IL-15), and is differentiated from naïve CD8+ T cells by Langerhans cells. RNA sequencing analysis identified that these CTLs express a gene signature that includes an array of surface receptors typically expressed by natural killer (NK) cells. We determined that CD8+ CTLs expressing granzyme B, perforin, and granulysin, as well as the activating NK receptor NKG2C, represent a population of "antimicrobial CTLs" (amCTLs) capable of T cell receptor (TCR)-dependent and TCR-independent release of cytotoxic granule proteins that mediate antimicrobial activity.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



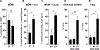
References
-
- Tan JS, Canaday DH, Boom WH, Balaji KN, Schwander SK, Rich EA, Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: Role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J. Immunol 159, 290–297 (1997). - PubMed
-
- Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat J-P, Sette A, Brenner MB, Porcelli SA, Bloom BR, Modlin RL, Differential effects of cytolytic T cell subsets on intracellular infection. Science 276, 1684–1687 (1997). - PubMed
-
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C, Porcelli SA, Bloom BR, Krensky AM, Modlin RL, An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282, 121–125 (1998). - PubMed
-
- Ochoa M-T, Stenger S, Sieling PA, Thoma-Uszynski S, Sabet S, Cho S, Krensky AM, Rollinghoff M, Sarno EN, Burdick AE, Rea TH, Modlin RL, T-cell release of granulysin contributes to host defense in leprosy. Nat. Med 7, 174–179 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

