Hepatitis B Virus Surface Antigen Enhances the Sensitivity of Hepatocytes to Fas-Mediated Apoptosis via Suppression of AKT Phosphorylation
- PMID: 30171166
- PMCID: PMC6176106
- DOI: 10.4049/jimmunol.1800732
Hepatitis B Virus Surface Antigen Enhances the Sensitivity of Hepatocytes to Fas-Mediated Apoptosis via Suppression of AKT Phosphorylation
Erratum in
-
Correction: Hepatitis B Virus Surface Antigen Enhances the Sensitivity of Hepatocytes to Fas-Mediated Apoptosis via Suppression of AKT Phosphorylation.J Immunol. 2020 Jul 1;205(1):300-301. doi: 10.4049/jimmunol.2000441. Epub 2020 Jun 1. J Immunol. 2020. PMID: 32482709 Free PMC article. No abstract available.
Abstract
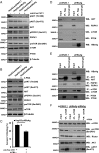
The Fas receptor/ligand system plays a prominent role in hepatic apoptosis and hepatocyte death. Although hepatitis B virus (HBV) surface Ag (HBsAg) is the most abundant HBV protein in the liver and peripheral blood of patients with chronic HBV infection, its role in Fas-mediated hepatocyte apoptosis has not been disclosed. In this study, we report that HBsAg sensitizes HepG2 cells to agonistic anti-Fas Ab CH11-induced apoptosis through increasing the formation of SDS-stable Fas aggregation and procaspase-8 cleavage but decreasing both the expression of cellular FLIPL/S and the recruitment of FLIPL/S at the death-inducing signaling complex (DISC). Notably, HBsAg increased endoplasmic reticulum stress and consequently reduced AKT phosphorylation by deactivation of phosphoinositide-dependent kinase-1 (PDPK1) and mechanistic target of rapamycin complex 2 (mTORC2), leading to enhancement of Fas-mediated apoptosis. In a mouse model, expression of HBsAg in mice injected with recombinant adenovirus-associated virus 8 aggravated Jo2-induced acute liver failure, which could be effectively attenuated by the AKT activator SC79. Based on these results, it is concluded that HBsAg predisposes hepatocytes to Fas-mediated apoptosis and mice to acute liver failure via suppression of AKT prosurviving activity, suggesting that interventions directed at enhancing the activation or functional activity of AKT may be of therapeutic value in Fas-mediated progressive liver cell injury and liver diseases.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures





References
-
- Nagata S. 1997. Apoptosis by death factor. Cell 88: 355–365. - PubMed
-
- Kondo T., Suda T., Fukuyama H., Adachi M., Nagata S. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3: 409–413. - PubMed
-
- Mochizuki K., Hayashi N., Hiramatsu N., Katayama K., Kawanishi Y., Kasahara A., Fusamoto H., Kamada T. 1996. Fas antigen expression in liver tissues of patients with chronic hepatitis B. J. Hepatol. 24: 1–7. - PubMed
-
- Luo K. X., Zhu Y. F., Zhang L. X., He H. T., Wang X. S., Zhang L. 1997. In situ investigation of Fas/FasL expression in chronic hepatitis B infection and related liver diseases. J. Viral Hepat. 4: 303–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

