Perfused 3D angiogenic sprouting in a high-throughput in vitro platform
- PMID: 30171498
- PMCID: PMC6510881
- DOI: 10.1007/s10456-018-9647-0
Perfused 3D angiogenic sprouting in a high-throughput in vitro platform
Abstract
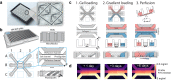
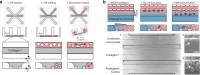
Angiogenic sprouting, the growth of new blood vessels from pre-existing vessels, is orchestrated by cues from within the cellular microenvironment, such as biochemical gradients and perfusion. However, many of these cues are missing in current in vitro models of angiogenic sprouting. We here describe an in vitro platform that integrates both perfusion and the generation of stable biomolecular gradients and demonstrate its potential to study more physiologically relevant angiogenic sprouting and microvascular stabilization. The platform consists of an array of 40 individually addressable microfluidic units that enable the culture of perfused microvessels against a three-dimensional collagen-1 matrix. Upon the introduction of a gradient of pro-angiogenic factors, the endothelial cells differentiated into tip cells that invaded the matrix. Continuous exposure resulted in continuous migration and the formation of lumen by stalk cells. A combination of vascular endothelial growth factor-165 (VEGF-165), phorbol 12-myristate 13-acetate (PMA), and sphingosine-1-phosphate (S1P) was the most optimal cocktail to trigger robust, directional angiogenesis with S1P being crucial for guidance and repetitive sprout formation. Prolonged exposure forces the angiogenic sprouts to anastomose through the collagen to the other channel. This resulted in remodeling of the angiogenic sprouts within the collagen: angiogenic sprouts that anastomosed with the other perfusion channel remained stable, while those who did not retracted and degraded. Furthermore, perfusion with 150 kDa FITC-Dextran revealed that while the angiogenic sprouts were initially leaky, once they fully crossed the collagen lane they became leak tight. This demonstrates that once anastomosis occurred, the sprouts matured and suggests that perfusion can act as an important survival and stabilization factor for the angiogenic microvessels. The robustness of this platform in combination with the possibility to include a more physiological relevant three-dimensional microenvironment makes our platform uniquely suited to study angiogenesis in vitro.
Keywords: 3D cell culture; Angiogenic sprouting; In vitro; Microfluidics; Vascular stabilization.
Conflict of interest statement
P. Vulto and T. Hankemeier are shareholders in Mimetas BV. V. van Duinen, D. Zhu, C. Ramakers and A. J. van Zonneveld declare no potential conflict of interest.
Figures


References
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. - PubMed
-
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16(2):196–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

