Effect of multiple-dose osimertinib on the pharmacokinetics of simvastatin and rosuvastatin
- PMID: 30171779
- PMCID: PMC6256010
- DOI: 10.1111/bcp.13753
Effect of multiple-dose osimertinib on the pharmacokinetics of simvastatin and rosuvastatin
Abstract
Aim: We report on two Phase 1, open-label, single-arm studies assessing the effect of osimertinib on simvastatin (CYP3A substrate) and rosuvastatin (breast cancer resistance protein substrate [BCRP] substrate) exposure in patients with advanced epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer who have progressed after treatment with an EGFR tyrosine kinase inhibitor, to determine, upon coadministration, whether osimertinib could affect the exposure of these agents.
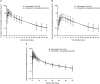
Methods: Fifty-two patients in the CYP3A study (pharmacokinetic [PK] analysis, n = 49), and 44 patients in the BCRP study were dosed (PK analysis, n = 44). In the CYP3A study, patients received single doses of simvastatin 40 mg on Days 1 and 31, and osimertinib 80 mg once daily on Days 3-32. In the BCRP study, single doses of rosuvastatin 20 mg were given on Days 1 and 32, and osimertinib 80 mg once daily on Days 4-34.
Results: Geometric least squares mean (GLSM) ratios (90% confidence intervals) of simvastatin plus osimertinib for area under the plasma concentration-time curves from zero to infinity (AUC) were 91% (77-108): entirely contained within the predefined no relevant effect limits, and Cmax of 77% (63, 94) which was not contained within the limits. GLSM ratios of rosuvastatin plus osimertinib for AUC were 135% (115-157) and Cmax were 172 (146, 203): outside the no relevant effect limits.
Conclusions: Osimertinib is unlikely to have any clinically relevant interaction with CYP3A substrates and has a weak inhibitory effect on BCRP. No new safety concerns were identified in either study.
Trial registration: ClinicalTrials.gov NCT02197234 NCT02317016.
Keywords: BCRP; CYP3A; NSCLC; osimertinib.
© 2018 The British Pharmacological Society.
Figures
References
-
- Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al Metastatic non‐small‐cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016; 27: v1–v27. - PubMed
-
- Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K, Peled N, et al The International Association for the Study of Lung Cancer consensus statement on optimizing management of EGFR mutation‐positive non‐small cell lung cancer: status in 2016. J Thorac Oncol 2016; 11: 946–963. - PubMed
-
- Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017; 35: 3484–3515. - PubMed
-
- Kuiper JL, Heideman DA, Thunnissen E, Paul MA, van Wijk AW, Postmus PE, et al Incidence of T790M mutation in (sequential) rebiopsies in EGFR‐mutated NSCLC‐patients. Lung Cancer 2014; 85: 19–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous