Somatic TP53 Mutations Are Detectable in Circulating Tumor DNA from Children with Anaplastic Wilms Tumors
- PMID: 30172241
- PMCID: PMC6121832
- DOI: 10.1016/j.tranon.2018.08.006
Somatic TP53 Mutations Are Detectable in Circulating Tumor DNA from Children with Anaplastic Wilms Tumors
Abstract
Background: Diffuse anaplastic Wilms tumor (DAWT) is a rare, high-risk subtype that is often missed on diagnostic needle biopsy. Somatic mutations in TP53 are associated with the development of anaplasia and with poorer survival, particularly in advanced-stage disease. Early identification of DAWT harboring TP53 abnormalities could improve risk stratification of initial therapy and monitoring for recurrence.
Methods: Droplet digital polymerase chain reaction (ddPCR) was used to evaluate 21 samples from 4 patients with DAWT. For each patient, we assessed TP53 status in frozen tumor, matched germline DNA, and circulating tumor DNA (ctDNA) from plasma, serum, and urine collected throughout treatment.
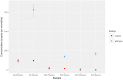
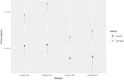
Results: Mutant TP53 was detectable in ctDNA from plasma and serum in all patients. We did not detect variant TP53 in the same volume (200 μl) of urine. One patient displayed heterogeneity of TP53 in the tumor despite both histological sections displaying anaplasia. Concentration of ctDNA from plasma/serum taken prenephrectomy varied significantly between patients, ranging from 0.44 (0.05-0.90) to 125.25 (109.75-140.25) copies/μl. We observed variation in ctDNA throughout treatment, and in all but one patient, ctDNA levels fell significantly following nephrectomy.
Conclusion: We demonstrate for the first time that ddPCR is an effective method for detection of mutant TP53 in ctDNA from children with DAWT even when there is intratumoral somatic heterogeneity. This should be further explored in a larger cohort of patients, as early detection of circulating variant TP53 may have significant clinical impact on future risk stratification and surveillance.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Monitoring Treatment Response, Early Recurrence, and Survival in Uterine Serous Carcinoma and Carcinosarcoma Patients Using Personalized Circulating Tumor DNA Biomarkers.Int J Mol Sci. 2023 May 17;24(10):8873. doi: 10.3390/ijms24108873. Int J Mol Sci. 2023. PMID: 37240216 Free PMC article.
-
TP53 mutational status is a potential marker for risk stratification in Wilms tumour with diffuse anaplasia.PLoS One. 2014 Oct 14;9(10):e109924. doi: 10.1371/journal.pone.0109924. eCollection 2014. PLoS One. 2014. PMID: 25313908 Free PMC article.
-
Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients.BMC Cancer. 2017 Jun 19;17(1):428. doi: 10.1186/s12885-017-3424-0. BMC Cancer. 2017. PMID: 28629339 Free PMC article.
-
Characteristics and outcomes of preoperatively treated patients with anaplastic Wilms tumors registered in the UK SIOP-WT-2001 and IMPORT study cohorts (2002-2020).Cancer. 2022 Apr 15;128(8):1666-1675. doi: 10.1002/cncr.34107. Epub 2022 Feb 4. Cancer. 2022. PMID: 35119702
-
Anaplasia in Wilms tumor: A critical review.Pediatr Blood Cancer. 2024 Jul;71(7):e31000. doi: 10.1002/pbc.31000. Epub 2024 Apr 11. Pediatr Blood Cancer. 2024. PMID: 38605554 Review.
Cited by
-
Biomarkers for patients with Wilms tumor: a review.Front Oncol. 2023 Jul 24;13:1137346. doi: 10.3389/fonc.2023.1137346. eCollection 2023. Front Oncol. 2023. PMID: 37554168 Free PMC article. Review.
-
A comprehensive overview of liquid biopsy applications in pediatric solid tumors.NPJ Precis Oncol. 2024 Aug 3;8(1):172. doi: 10.1038/s41698-024-00657-z. NPJ Precis Oncol. 2024. PMID: 39097671 Free PMC article. Review.
-
Hallmark discoveries in the biology of Wilms tumour.Nat Rev Urol. 2024 Mar;21(3):158-180. doi: 10.1038/s41585-023-00824-0. Epub 2023 Oct 17. Nat Rev Urol. 2024. PMID: 37848532 Review.
-
TP53 mutation and MET amplification in circulating tumor DNA analysis predict disease progression in patients with advanced gastric cancer.PeerJ. 2021 Apr 16;9:e11146. doi: 10.7717/peerj.11146. eCollection 2021. PeerJ. 2021. PMID: 33959414 Free PMC article.
-
Liquid biopsies in pediatric oncology: opportunities and obstacles.Curr Opin Pediatr. 2022 Feb 1;34(1):39-47. doi: 10.1097/MOP.0000000000001088. Curr Opin Pediatr. 2022. PMID: 34840249 Free PMC article. Review.
References
-
- Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172–181. - PubMed
-
- van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, van Tinteren H, Furtwängler R, Verschuur AC, Vujanic GM, Leuschner I, Brok J, Rübe C. Position paper: rationale for the treatment of Wilms tumour in the UMBRELLA SIOP–RTSG 2016 protocol. Nat Rev Urol. 2017;14:743–752. - PubMed
-
- Pritchard-Jones K, Bergeron C, de Camargo B, van den Heuvel-Eibrink MM, Acha T, Godzinski J, Oldenburger F, Boccon-Gibod L, Leuschner I, Vujanic G. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms' tumour (SIOP WT 2001): an open-label, non-inferiority, randomised controlled trial. Lancet. 2015;386:1156–1164. - PubMed
-
- Vujanić GM, Kelsey A, Mitchell C, Shannon RS, Gornall P. The role of biopsy in the diagnosis of renal tumors of childhood: Results of the UKCCSG Wilms tumor study 3. Med Pediatr Oncol. 2003;40:18–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

