Purified CD34+ cells versus peripheral blood mononuclear cells in the treatment of angiitis-induced no-option critical limb ischaemia: 12-Month results of a prospective randomised single-blinded non-inferiority trial
- PMID: 30172703
- PMCID: PMC6156701
- DOI: 10.1016/j.ebiom.2018.08.038
Purified CD34+ cells versus peripheral blood mononuclear cells in the treatment of angiitis-induced no-option critical limb ischaemia: 12-Month results of a prospective randomised single-blinded non-inferiority trial
Abstract
Background: Peripheral blood mononuclear cells (PBMNCs) and purified CD34+ cells (PCCs) are increasingly being used at treating no-option critical limb ischaemia (NO-CLI). We aimed to compare the efficacies and uncover the advantages associated with each treatment approach.
Methods: A randomised single-blinded non-inferiority trial (Number: NCT 02089828) was performed. NO-CLI patients were 1:1 randomised to the PBMNCs and PCCs groups, and compared in relation to safety and efficacy outcomes. The primary efficacy outcomes included major amputation and total amputation over 12 months. The major amputation-free survival (MAFS) and total amputation-free survival (TAFS) rates were calculated.
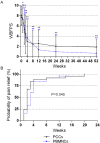
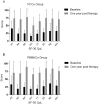
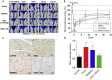
Findings: Fifty patients (25 per group, 47 with thromboangiitis obliterans and 3 with other angiitis) were enrolled, with a median follow-up period of 24.5 months (interquartile range: 17-34 months). One patient in the PCCs group was lost at 2 months and one major amputation occurred in the PBMNCs group at 3 months post-transplantation. The total amputation rates at 6 months post-transplantation were 28.0% in the PCCs group and 16.0% in the PBMNCs group (p = 0.343), and remained unchanged at 12 months. The groups did not differ regarding the MAFS and TAFS (Breslow-Wilcoxon test: p = 0.3014 and p = 0.3414). The PCCs group had a significantly higher probability of rest pain relief than the PBMNCs group (Breslow-Wilcoxon test: p = 0.0454).
Interpretation: PCCs was not inferior to PBMNCs at limb salvage in the treatment of angiitis-induced NO-CLI and appeared to induce earlier ischaemia relief. Each cell type had specific advantages. These outcomes require verification from longer-term trials involving larger numbers of patients. FUND: Training program for outstanding academic leaders of Shanghai health and family planning system (Hundred Talent Program,Grant No. 2018BR40); China National Natural Science Funds (Grant No. 30801122); The excellent core member training programme at Zhongshan Hospital, Fudan University, China (Grant No. 2015ZSYXGG02); and Zhongshan Funds for the Institute of Vascular Surgery, Fudan University, China.
Clinical trial registration: This study is registered with ClinicalTrials.gov (NCT 02089828).
Trial registration: ClinicalTrials.gov NCT02089828.
Keywords: Cell therapy; Critical limb ischaemia; Limb salvage; Peripheral blood mononuclear cells; Purified CD34(+) cells.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Autologous peripheral blood-derived stem cells transplantation for treatment of no-option angiitis-induced critical limb ischemia: 10-year management experience.Stem Cell Res Ther. 2020 Oct 28;11(1):458. doi: 10.1186/s13287-020-01981-4. Stem Cell Res Ther. 2020. PMID: 33115517 Free PMC article. Clinical Trial.
-
The peripheral blood mononuclear cells versus purified CD34+ cells transplantation in patients with angiitis-induced critical limb ischemia trial: 5-year outcomes and return to work analysis-a randomized single-blinded non-inferiority trial.Stem Cell Res Ther. 2022 Mar 21;13(1):116. doi: 10.1186/s13287-022-02804-4. Stem Cell Res Ther. 2022. PMID: 35313967 Free PMC article. Clinical Trial.
-
Three-year outcomes of peripheral blood mononuclear cells vs purified CD34+ cells in the treatment of angiitis-induced no-option critical limb ischemia and a cost-effectiveness assessment: A randomized single-blinded noninferiority trial.Stem Cells Transl Med. 2021 May;10(5):647-659. doi: 10.1002/sctm.20-0033. Epub 2021 Jan 5. Stem Cells Transl Med. 2021. PMID: 33399273 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis.Curr Stem Cell Res Ther. 2018;13(4):265-283. doi: 10.2174/1574888X13666180313141416. Curr Stem Cell Res Ther. 2018. PMID: 29532760 Review.
-
Towards a more relevant hind limb model of muscle ischaemia.Atherosclerosis. 2013 Mar;227(1):1-8. doi: 10.1016/j.atherosclerosis.2012.10.060. Epub 2012 Nov 2. Atherosclerosis. 2013. PMID: 23177969 Review.
Cited by
-
Long-Term Outcomes of Peripheral Blood Mononuclear Cells in the Treatment of Angiitis-Induced No-Option Critical Limb-Threatening Ischemia.Front Cardiovasc Med. 2021 Dec 6;8:769472. doi: 10.3389/fcvm.2021.769472. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34938786 Free PMC article.
-
Therapeutic Angiogenesis Using Autologous CD34-Positive Cells for Vascular Diseases.Ann Vasc Dis. 2022 Dec 25;15(4):241-252. doi: 10.3400/avd.ra.22-00086. Ann Vasc Dis. 2022. PMID: 36644256 Free PMC article.
-
Autologous peripheral blood-derived stem cells transplantation for treatment of no-option angiitis-induced critical limb ischemia: 10-year management experience.Stem Cell Res Ther. 2020 Oct 28;11(1):458. doi: 10.1186/s13287-020-01981-4. Stem Cell Res Ther. 2020. PMID: 33115517 Free PMC article. Clinical Trial.
-
Return to work after cell transplantation in patients with angiitis-induced critical limb ischaemia and factors related: a single-centre retrospective cohort study.Stem Cell Res Ther. 2022 Apr 1;13(1):139. doi: 10.1186/s13287-022-02807-1. Stem Cell Res Ther. 2022. PMID: 35365238 Free PMC article.
-
Etrinabdione (VCE-004.8), a B55α activator, promotes angiogenesis and arteriogenesis in critical limb ischemia.J Transl Med. 2024 Nov 6;22(1):1003. doi: 10.1186/s12967-024-05748-w. J Transl Med. 2024. PMID: 39506809 Free PMC article.
References
-
- Dormandy J., Heeck L., Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. - PubMed
-
- Lawall H., Bramlage P., Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. - PubMed
-
- Norgren L., Hiatt W.R., Dormandy J.A. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):5–67. - PubMed
-
- Powell R.J. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56:264–266. - PubMed
-
- Minamino T., Toko H., Tateno K., Nagai T., Komuro I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet. 2002;360:2083–2084. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

