Resetting the Stress System with a Mifepristone Challenge
- PMID: 30173378
- PMCID: PMC6469632
- DOI: 10.1007/s10571-018-0614-5
Resetting the Stress System with a Mifepristone Challenge
Abstract
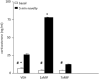
Psychotic depression is characterized by elevated circulating cortisol, and high daily doses of the glucocorticoid/progesterone antagonist mifepristone for 1 week are required for significant improvement. Using a rodent model, we find that such high doses of mifepristone are needed because the antagonist is rapidly degraded and poorly penetrates the blood-brain barrier, but seems to facilitate the entry of cortisol. We also report that in male C57BL/6J mice, after a 7-day treatment with a high dose of mifepristone, basal blood corticosterone levels were similar to that of vehicle controls. This is surprising because after the first mifepristone challenge, corticosterone remained elevated for about 16 h, and then decreased towards vehicle control levels at 24 h. At that time, stress-induced corticosterone levels of the 1xMIF were sevenfold higher than the 7xMIF group, the latter response being twofold lower than controls. The 1xMIF mice showed behavioral hyperactivity during exploration of the circular hole board, while the 7xMIF mice rather engaged in serial search patterns. To explain this rapid reset of corticosterone secretion upon recurrent mifepristone administration, we suggest the following: (i) A rebound glucocorticoid feedback after cessation of mifepristone treatment. (ii) Glucocorticoid agonism in transrepression and recruitment of cell-specific coregulator cocktails. (iii) A more prominent role of brain MR function in control of stress circuit activity. An overview table of neuroendocrine MIF effects is provided. The data are of interest for understanding the mechanistic underpinning of stress system reset as treatment strategy for stress-related diseases.
Keywords: Behavior; Brain; Glucocorticoid receptor; Mineralocorticoid receptor; RU38486; Stress.
Conflict of interest statement
SD and AMK report no conflict of interest. ERdK is on the Scientific Advisory Board of the DynaCorts Group and owns stock of Corcept Therapeutics. OCM receives funding from Corcept Therapeutics. JKB is an employee of Corcept Therapeutics, which develops novel GR ligands.
Figures






References
-
- Arp JM, ter Horst JP, Loi M et al (2016) Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol Learn Mem 133:30–38. 10.1016/j.nlm.2016.05.009 - PubMed
-
- Atucha E, Zalachoras I, van den Heuvel JK et al (2015) A mixed glucocorticoid/mineralocorticoid selective modulator with dominant antagonism in the male rat brain. Endocrinology 156:4105–4114. 10.1210/en.2015-1390 - PubMed
-
- Bachmann CG, Linthorst ACE, Holsboer F, Reul JMHM (2003) Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology 28:1056–1067. 10.1038/sj.npp.1300158 - PubMed
-
- Belanoff JK, Flores BH, Kalezhan M et al (2001) Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol 21:516–521. 10.1097/00004714-200110000-00009 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

