Virus-like particle vaccines: immunology and formulation for clinical translation
- PMID: 30173619
- PMCID: PMC7103734
- DOI: 10.1080/14760584.2018.1516552
Virus-like particle vaccines: immunology and formulation for clinical translation
Abstract
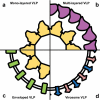
Introduction: Virus-like particle (VLP) vaccines face significant challenges in their translation from laboratory models, to routine clinical administration. While some VLP vaccines thrive and are readily adopted into the vaccination schedule, others are restrained by regulatory obstacles, proprietary limitations, or finding their niche amongst the crowded vaccine market. Often the necessity to supplant an existing vaccination regimen possesses an immediate obstacle for the development of a VLP vaccine, despite any preclinical advantages identified over the competition. Novelty, adaptability and formulation compatibility may prove invaluable in helping place VLP vaccines at the forefront of vaccination technology.
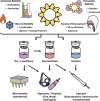
Areas covered: The purpose of this review is to outline the diversity of VLP vaccines, VLP-specific immune responses, and to explore how modern formulation and delivery techniques can enhance the clinical relevance and overall success of VLP vaccines.
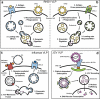
Expert commentary: The role of formation science, with an emphasis on the diversity of immune responses induced by VLP, is underrepresented amongst clinical trials for VLP vaccines. Harnessing such diversity, particularly through the use of combinations of select excipients and adjuvants, will be paramount in the development of VLP vaccines.
Keywords: Clinical translation; VLP; formulation; immunology; vaccine; virus-like particle.
Figures
References
-
- Al-Barwani F, Donaldson B, Pelham SJ, et al. Antigen delivery by virus-like particles for immunotherapeutic vaccination. Ther Deliv. 2014. November;5(11):1223–1240. PubMed PMID: 25491672. - PubMed
-
• This review covers the structural diversity of VLP, post-production modification, and the immune response to VLP vaccines with a specific focus on immunotherapeutic vaccine development.
-
- Lachance P, Dionne M, Libman M, et al, Medicago, Inc; Immunogenicity of a quadrivalent virus-like particles (VLP) influenza vaccine in healthy adults. NCT02768805. 2016.
-
- Sheldon E, Seiden DJ, Medicago, Inc; Immunogenicity, safety and tolerability of a plant-derived seasonal virus-like-particle quadrivalent influenza vaccine in adults. NCT02233816. 2016.
-
- Acevedo-Flores M, Diaz C, Hoen B, et al, National Institute of Allergy and Infectious Diseases (NIAID) Trial for safety and immunogenicity of a chikungunya vaccine, VRC-CHKVLP059-00-VP, in healthy adults. NCT02562482. 2016.
-
- Langley J, VBI Vaccines Inc; Study to evaluate safety, tolerability, and immunogenicity of candidate human cytomegalovirus vaccine in healthy adults. NCT02826798. 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
