A conceptual model for optimizing vaccine coverage to reduce vector-borne infections in the presence of antibody-dependent enhancement
- PMID: 30173664
- PMCID: PMC6120075
- DOI: 10.1186/s12976-018-0085-x
A conceptual model for optimizing vaccine coverage to reduce vector-borne infections in the presence of antibody-dependent enhancement
Abstract
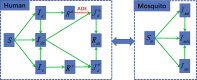
Background: Many vector-borne diseases co-circulate, as the viruses from the same family are also transmitted by the same vector species. For example, Zika and dengue viruses belong to the same Flavivirus family and are primarily transmitted by a common mosquito species Aedes aegypti. Zika outbreaks have also commonly occurred in dengue-endemic areas, and co-circulation and co-infection of both viruses have been reported. As recent immunological cross-reactivity studies have confirmed that convalescent plasma following dengue infection can enhance Zika infection, and as global efforts of developing dengue and Zika vaccines are intensified, it is important to examine whether and how vaccination against one disease in a large population may affect infection dynamics of another disease due to antibody-dependent enhancement.
Methods: Through a conceptual co-infection dynamics model parametrized by reported dengue and Zika epidemic and immunological cross-reactivity characteristics, we evaluate impact of a hypothetical dengue vaccination program on Zika infection dynamics in a single season when only one particular dengue serotype is involved.
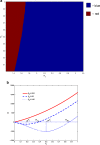
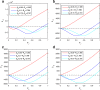
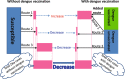
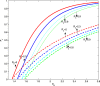
Results: We show that an appropriately designed and optimized dengue vaccination program can not only help control the dengue spread but also, counter-intuitively, reduce Zika infections. We identify optimal dengue vaccination coverages for controlling dengue and simultaneously reducing Zika infections, as well as the critical coverages exceeding which dengue vaccination will increase Zika infections.
Conclusion: This study based on a conceptual model shows the promise of an integrative vector-borne disease control strategy involving optimal vaccination programs, in regions where different viruses or different serotypes of the same virus co-circulate, and convalescent plasma following infection from one virus (serotype) can enhance infection against another virus (serotype). The conceptual model provides a first step towards well-designed regional and global vector-borne disease immunization programs.
Keywords: Antibody dependent enhancement; Dengue; Mathematical modelling; Optimized vaccination strategies; Zika.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Current concerns and perspectives on Zika virus co-infection with arboviruses and HIV.J Autoimmun. 2018 May;89:11-20. doi: 10.1016/j.jaut.2018.01.002. Epub 2018 Jan 17. J Autoimmun. 2018. PMID: 29352633 Free PMC article. Review.
-
Estimating per-infection cost and burden for dengue and Zika as a function of antibody-dependent enhancement.PLoS Negl Trop Dis. 2025 Feb 27;19(2):e0012876. doi: 10.1371/journal.pntd.0012876. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 40014622 Free PMC article.
-
Implication of sexual transmission of Zika on dengue and Zika outbreaks.Math Biosci Eng. 2019 Jun 3;16(5):5092-5113. doi: 10.3934/mbe.2019256. Math Biosci Eng. 2019. PMID: 31499705
-
Cross-Protection Against Four Serotypes of Dengue Virus in Mice Conferred by a Zika DNA Vaccine.Front Cell Infect Microbiol. 2019 May 8;9:147. doi: 10.3389/fcimb.2019.00147. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31139577 Free PMC article.
-
The Challenges Imposed by Dengue, Zika, and Chikungunya to Brazil.Front Immunol. 2018 Aug 28;9:1964. doi: 10.3389/fimmu.2018.01964. eCollection 2018. Front Immunol. 2018. PMID: 30210503 Free PMC article. Review.
Cited by
-
Modelling the impact of antibody-dependent enhancement on disease severity of Zika virus and dengue virus sequential and co-infection.R Soc Open Sci. 2020 Apr 15;7(4):191749. doi: 10.1098/rsos.191749. eCollection 2020 Apr. R Soc Open Sci. 2020. PMID: 32431874 Free PMC article.
-
Exploring Zika's dynamics: A scoping review journey from epidemic to equations through mathematical modelling.Infect Dis Model. 2024 Dec 31;10(2):536-558. doi: 10.1016/j.idm.2024.12.016. eCollection 2025 Jun. Infect Dis Model. 2024. PMID: 39897087 Free PMC article. Review.
-
The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age.Sci Rep. 2020 Oct 6;10(1):16642. doi: 10.1038/s41598-020-73777-8. Sci Rep. 2020. PMID: 33024235 Free PMC article.
References
-
- World Health Organization (WHO). WHO Dengue and Severe Dengue, Fact Sheet No. 117, Updated May 2015. http://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue.
Publication types
MeSH terms
Substances
Grants and funding
- 11571273, 11631012/National Natural Science Foundation of China/International
- 08143042/Fundamental Research Funds for the Central Universities/International
- DMS-1412454/National Science Foundation/International
- 230720/Canada Excellence Research Chairs, Government of Canada/International
- 446610-3/Natural Sciences and Engineering Research Council of Canada/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

