Light Affects Mood and Learning through Distinct Retina-Brain Pathways
- PMID: 30173913
- PMCID: PMC6190605
- DOI: 10.1016/j.cell.2018.08.004
Light Affects Mood and Learning through Distinct Retina-Brain Pathways
Abstract
Light exerts a range of powerful biological effects beyond image vision, including mood and learning regulation. While the source of photic information affecting mood and cognitive functions is well established, viz. intrinsically photosensitive retinal ganglion cells (ipRGCs), the central mediators are unknown. Here, we reveal that the direct effects of light on learning and mood utilize distinct ipRGC output streams. ipRGCs that project to the suprachiasmatic nucleus (SCN) mediate the effects of light on learning, independently of the SCN's pacemaker function. Mood regulation by light, on the other hand, requires an SCN-independent pathway linking ipRGCs to a previously unrecognized thalamic region, termed perihabenular nucleus (PHb). The PHb is integrated in a distinctive circuitry with mood-regulating centers and is both necessary and sufficient for driving the effects of light on affective behavior. Together, these results provide new insights into the neural basis required for light to influence mood and learning.
Keywords: aberrant light cycle; circadian rhythms; ipRGCs; learning; mood; perihabenular nucleus; suprachiasmatic nucleus; ventromedial prefrontal cortex.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing financial conflicts of interest.
Figures
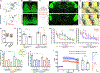
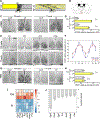
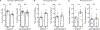


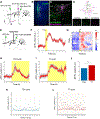

Comment in
-
A Thalamic Circuit Lights up Mood.Cell. 2018 Sep 20;175(1):34-35. doi: 10.1016/j.cell.2018.08.054. Cell. 2018. PMID: 30241611
References
-
- Bedrosian TA, Fonken LK, Walton JC, Haim A, and Nelson RJ (2011). Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36, 1062–1069. - PubMed
-
- Berson DM, Dunn FA, and Takao M (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

