Memory B Cells Activate Brain-Homing, Autoreactive CD4+ T Cells in Multiple Sclerosis
- PMID: 30173916
- PMCID: PMC6191934
- DOI: 10.1016/j.cell.2018.08.011
Memory B Cells Activate Brain-Homing, Autoreactive CD4+ T Cells in Multiple Sclerosis
Abstract
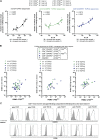
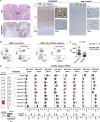
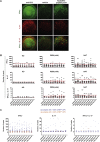
Multiple sclerosis is an autoimmune disease that is caused by the interplay of genetic, particularly the HLA-DR15 haplotype, and environmental risk factors. How these etiologic factors contribute to generating an autoreactive CD4+ T cell repertoire is not clear. Here, we demonstrate that self-reactivity, defined as "autoproliferation" of peripheral Th1 cells, is elevated in patients carrying the HLA-DR15 haplotype. Autoproliferation is mediated by memory B cells in a HLA-DR-dependent manner. Depletion of B cells in vitro and therapeutically in vivo by anti-CD20 effectively reduces T cell autoproliferation. T cell receptor deep sequencing showed that in vitro autoproliferating T cells are enriched for brain-homing T cells. Using an unbiased epitope discovery approach, we identified RASGRP2 as target autoantigen that is expressed in the brain and B cells. These findings will be instrumental to address important questions regarding pathogenic B-T cell interactions in multiple sclerosis and possibly also to develop novel therapies.
Keywords: B cells; HLA-DR15; RASGRP2; T cell receptor; T cells; autoproliferation; brain; multiple sclerosis; pathogenesis.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
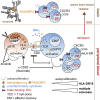







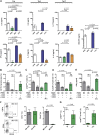
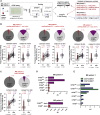
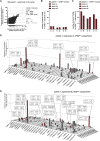

Comment in
-
Immune-cell crosstalk in multiple sclerosis.Nature. 2018 Nov;563(7730):194-195. doi: 10.1038/d41586-018-07063-z. Nature. 2018. PMID: 30390068 No abstract available.
References
-
- Basdeo S.A., Cluxton D., Sulaimani J., Moran B., Canavan M., Orr C., Veale D.J., Fearon U., Fletcher J.M. Ex-Th17 (Nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J. Immunol. 2017;198:2249–2259. - PubMed
-
- Beecham A.H., Patsopoulos N.A., Xifara D.K., Davis M.F., Kemppinen A., Cotsapas C., Shah T.S., Spencer C., Booth D., Goris A., International Multiple Sclerosis Genetics Consortium (IMSGC) Wellcome Trust Case Control Consortium 2 (WTCCC2) International IBD Genetics Consortium (IIBDGC) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 2013;45:1353–1360. - PMC - PubMed
-
- Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. - PubMed
-
- Cai G., Hafler D.A. Multispecific responses by T cells expanded by endogenous self-peptide/MHC complexes. Eur. J. Immunol. 2007;37:602–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

