An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B
- PMID: 30174468
- PMCID: PMC6110283
- DOI: 10.2147/JBM.S159297
An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B
Abstract
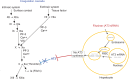
Fitusiran is an RNA interference therapeutic that targets antithrombin (AT) in the liver and interferes with AT translation by binding and degrading messenger RNA-AT, thereby silencing AT gene expression and preventing AT synthesis. In both preclinical and clinical studies, AT knockdown results in dose-dependent AT lowering when fitusiran is given weekly or monthly subcutaneously. In clinical trials, fitusiran dose escalation has resulted in improved thrombin generation and clinical hemostasis as measured by reduction in annualized bleed rate. Unlike currently licensed drugs, this improvement was not only in patients with hemophilia A but in also those with hemophilia B, with or without inhibitors. In dental and surgical procedures, fitusiran also provided perioperative hemostasis in association with AT lowering. Fitusiran is well tolerated, with minor local injection site reactions, but in one subject with severe hemophilia A, the concomitant use of daily high-dose factor VIII, inconsistent with trial guidance to avoid high, repeat doses of clotting factor, was associated with fatal thrombosis, suggesting the need for caution when using hemostatic agents in conjunction with fitusiran. Preclinical in vitro and in silico studies indicate improvement in thrombin generation in rare bleeding disorder plasmas, including in plasmas from patients with severe factors V, VII, and X deficiency, suggesting potential therapeutic benefit.
Keywords: clotting factor; congenital bleeding disorder; fitusiran; mRNA; novel bypass.
Conflict of interest statement
Disclosure Both authors report no conflicts of interest in this work.
Figures
References
-
- Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779. - PubMed
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. - PubMed
-
- Oladapo AO, Epstein JD, Williams E, Ito D, Gringeri A, Valentino LA. Health-related quality of life assessment in haemophilia patients on prophylaxis therapy: a systematic review of results from prospective clinical trials. Haemophilia. 2015;21(5):e344–e358. - PubMed
-
- Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046–4055. - PubMed
-
- Shuey DJ, Mccallus DE, Giordano T. RNAi: gene-silencing in therapeutic intervention. Drug Discov Today. 2002;7(20):1040–1046. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

