New Corticopontine Connections in the Primate Brain: Contralateral Projections From the Arm/Hand Area of the Precentral Motor Region
- PMID: 30174591
- PMCID: PMC6107685
- DOI: 10.3389/fnana.2018.00068
New Corticopontine Connections in the Primate Brain: Contralateral Projections From the Arm/Hand Area of the Precentral Motor Region
Abstract
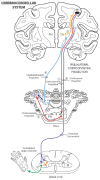
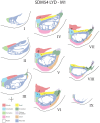
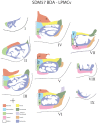
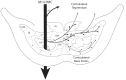
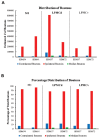
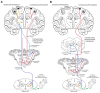
The ipsilateral corticopontine projection (iCPP) represents a massive descending axon system terminating in the pontine nuclei (PN). In the primate, this projection is well known for its dominant influence on contralateral upper limb movements through the classical cerebrocerebellar circuity system. Although a much weaker contralateral corticopontine projection (cCPP) from motor cortex to the paramedian region has been reported in the non-human primate brain, we provide the first comprehensive description of the cCPP from the lateral motor cortex using high resolution anterograde tract tracing in Macaca mulatta. We found a relatively light cCPP from the hand/arm area of the primary motor cortex (M1), comparatively moderate cCPP from ventrolateral premotor cortex (LPMCv) and a more robust and widespread cCPP from the dorsolateral premotor cortex (LPMCd) that involved all nine contralateral PN. The M1 projection primarily targeted the dorsal pontine region, the LPMCv projection targeted the medial pontine region and LPMCd targeted both regions. These results show the first stage of the primate frontomotor cerebrocerebellar projection is bilateral, and may affect both ipsilateral and contralateral limbs. Clinically, the cCPP originating in the non-injured hemisphere may influence the recovery process of the more affected upper extremity following subtotal unilateral damage to the lateral cortical region. The cCPP may also contribute to the mild impairment of the upper limb contralateral to a unilateral cerebellar injury.
Keywords: cerebellum; cerebrocebellar; corticofugal; frontal lobe; hand coordination; pons; pyramidal tract.
Figures






References
-
- Afifi A. K., Bergman R. A. (1980). Basic Neuroscience. Baltimore, MD: Urban and Schwarzenberg.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

