High-Throughput Identification of Candidate Strains for Biopreservation by Using Bioluminescent Listeria monocytogenes
- PMID: 30174662
- PMCID: PMC6107680
- DOI: 10.3389/fmicb.2018.01883
High-Throughput Identification of Candidate Strains for Biopreservation by Using Bioluminescent Listeria monocytogenes
Abstract
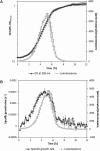
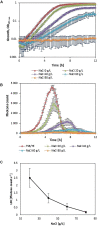
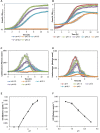
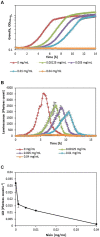
This article describes a method for high-throughput competition assays using a bioluminescent strain of L. monocytogenes. This method is based on the use of the luminescent indicator strain L. monocytogenes EGDelux. The luminescence of this strain is correlated to growth, which make it suitable to monitor the growth of L. monocytogenes in mixed cultures. To this aim, luminescence kinetics were converted into a single numerical value, called the Luminescence Disturbance Indicator (LDI), which takes into account growth inhibition phenomena resulting in latency increase, decrease in the luminescence rate, or reduction of the maximum luminescence. The LDI allows to automatically and simultaneously handle multiple competition assays which are required for high-throughput screening (HTS) approaches. The method was applied to screen a collection of 1810 strains isolated from raw cow's milk in order to identify non-acidifying strains with anti-L. monocytogenes bioprotection properties. This method was also successfully used to identify anti-L. monocytogenes candidates within a collection of Lactococcus piscium, a species where antagonism was previously described as non-diffusible and requiring cell-to-cell contact. In conclusion, bioluminescent L. monocytogenes can be used in HTS to identify strains with anti-L. monocytogenes bioprotection properties, irrespectively of the inhibition mechanism.
Keywords: Listeria monocytogenes; antibacterial activities; bioluminescence; biopreservation; bioprotection; competition; high-throughput screening assays; mixed culture.
Figures

References
-
- Barth M., Hankinson T. R., Zhuang H., Breidt F. (2009). “Microbiological spoilage of fruits and vegetables,” in Compendium of the Microbiological Spoilage of Foods and Beverages Food Microbiology and Food Safety, eds Sperber W., Doyle M. (New York, NY: Springer; ), 135–183. 10.1007/978-1-4419-0826-1_6 - DOI
-
- Bian X., Evivie S. E., Muhammad Z., Luo G.-W., Liang H.-Z., Wang N.-N., et al. (2016). In vitro assessment of the antimicrobial potentials of Lactobacillus helveticus strains isolated from traditional cheese in Sinkiang China against food-borne pathogens. Food Funct. 7 789–797. 10.1039/C5FO01041A - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

