Metabolomic analysis of uterine serous carcinoma with acquired resistance to paclitaxel
- PMID: 30174791
- PMCID: PMC6112827
- DOI: 10.18632/oncotarget.25868
Metabolomic analysis of uterine serous carcinoma with acquired resistance to paclitaxel
Erratum in
-
Correction: Metabolomic analysis of uterine serous carcinoma with acquired resistance to paclitaxel.Oncotarget. 2021 Nov 9;12(23):2321-2322. doi: 10.18632/oncotarget.27474. eCollection 2021 Nov 9. Oncotarget. 2021. PMID: 34786186 Free PMC article.
Abstract
Introduction: Uterine serous carcinoma (USC) is more aggressive than other subtypes of endometrial carcinoma and is associated with a poor prognosis. We analyzed the metabolomic profile of USC with acquired resistance to paclitaxel.
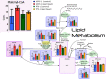
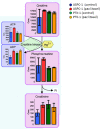
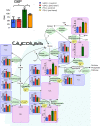
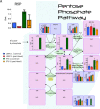
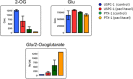
Results: Glutathione (GSH) concentration in PTX-1 cells was higher than in USPC-1 cells. In addition, GSH concentration in the USPC-1 cells increased after treatment with paclitaxel but was unchanged in PTX-1 cells. Glucose-6-phosphate (G6P) and ribose-5-phosphate (R5P) concentrations in PTX-1 cells were higher than those in USPC-1 cells. G6P concentration in the USPC-1 cells was unchanged after treatment with paclitaxel, while it decreased in PTX-1 cells.
Conclusion: Our results indicate that increased GSH and glucose metabolism may be related to acquiring resistance to paclitaxel in USC and thus may be targets for anti-USC therapy.
Materials and methods: We compared metabolic profiles and reactions to paclitaxel in both a wild type USC cell line (USPC-1) and PTX-1, a cell line derived from USPC-1 which acquired paclitaxel resistance, using a capillary electrophoresis CE-MS/MS system.
Keywords: endometrial cancer; metabolomic analysis; paclitaxel; uterine serous carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The Authors declare no conflicts of interest.
Figures



References
-
- Tropé C, Kristensen GB, Abeler VM. Clear-cell and papillary serous cancer: treatment options. Best Pract Res Clin Obstet Gynaecol. 2001;15:433–46. - PubMed
-
- Matthews RP, Hutchinson-Colas J, Maiman M, Fruchter RG, Gates EJ, Gibbon D, Remy JC, Sedlis A. Papillary serous and clear cell type lead to poor prognosis of endometrial carcinoma in black women. Gynecol Oncol. 1997;65:206–12. - PubMed
-
- Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, Oh JC, Atkinson EN, Broaddus RR, Gershenson DM, Lu KH. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol. 2003;91:463–69. - PubMed
-
- Podratz KC, Mariani A. Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol. 2003;91:461–62. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

