Assessment of Treatment Response Following Yttrium-90 Transarterial Radioembolization of Liver Malignancies
- PMID: 30175001
- PMCID: PMC6116887
- DOI: 10.7759/cureus.2895
Assessment of Treatment Response Following Yttrium-90 Transarterial Radioembolization of Liver Malignancies
Abstract
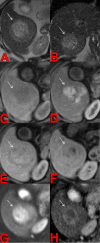
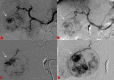
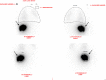
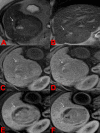
Transarterial radioembolization using yttrium-90 microspheres is an established and effective treatment for liver malignancies. Determining response to this treatment is difficult due to the radical changes that occur in tissue as a response to radiation. Though accurate assessment of treatment response is paramount for proper patient disposition, there is currently no standardized assessment protocol. Current methods of assessment often consider changes in size, necrosis, vascularity, fluorodeoxyglucose-positron emission tomography FDG-PET metabolic activity, and diffusion using diffusion-weighted magnetic resonance imaging (DWI). Current methods of assessment require a lag time of one to two months post-treatment to determine treatment effectiveness. This delay is a hindrance to obtaining better patient outcomes, giving rise to a need to identify markers for faster determination of treatment efficacy.
Keywords: brachytherapy; fdg-pet; liver cancer; malignant tumour; mri; post-treatment evaluation; radiation therapy; tumour necrosis; tumour vascularity; y90 transarterial radioembolization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Cancer statistics, 2016. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- ACR appropriateness criteria radiologic management of hepatic malignancy. Kouri BE, Funaki BS, Ray CE Jr, et al. J Am Coll Radiol. 2012;9:919–925. - PubMed
-
- Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. Keppke AL, Salem R, Reddy D, et al. AJR Am J Roentgenol. 2007;188:768–775. - PubMed
-
- Radiographically identified necrosis after 90Y microsphere brachytherapy: a new standard for oncologic response assessment? Welsh JS. AJR Am J Roentgenol. 2007;188:765–767. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
