Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications
- PMID: 30175095
- PMCID: PMC6108306
- DOI: 10.3389/fbioe.2018.00109
Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications
Abstract
Polydopamine (PDA), the final oxidation product of dopamine or other catecholamines, attracted much attention as versatile coatings that can be used to cover the surface of almost all materials with a conformal layer of adjustable thickness ranging from a few to about 100 nm. These PDA layers can be subsequently modified with molecules carrying nucleophilic groups or with metallic nanoparticles from solutions containing metallic cations. However, during the deposition of PDA film on the surfaces, the reaction products that are simultaneously obtained from the oxidation of catecholamines in solution precipitate. Hence, some recent effort has been devoted to produce PDA in the form of nanoparticles. The aim of this short review is to give a comprehensive description of the synthesis methods yielding of PDA nanoparticles in the absence or in the presence of templating agents (polymers, polyelectrolytes, surfactants, proteins, and even some small organic molecules). We will also describe the use of thin PDA layers to coat already synthesized nanoparticles or nanotubes. Finally, several first applications of the obtained PDA nanoparticles will be described.
Keywords: nanotubes; polydopamine; polydopamine nanoparticles; polyelectrolytes; proteins; surfactants.
Figures
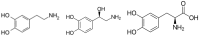
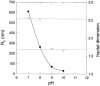
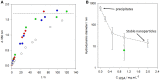
 ) and fractal dimension (right hand vertical scale,
) and fractal dimension (right hand vertical scale,  ) of eumelanin grains obtained at the end of the reaction kinetics from L-DOPA solutions, as function of the pH. Reproduced from Bridelli (1998) with authorization.
) of eumelanin grains obtained at the end of the reaction kinetics from L-DOPA solutions, as function of the pH. Reproduced from Bridelli (1998) with authorization.
 ) and in the presence of HAS at various concentrations: 0.2 mg.mL−1 (
) and in the presence of HAS at various concentrations: 0.2 mg.mL−1 ( ), 1 mg.mL−1 (
), 1 mg.mL−1 ( ), and 2 mg.mL−1 (
), and 2 mg.mL−1 ( ). The horizontal dashed line corresponds to the saturation absorption at the end of the oxidation kinetics of dopamine. (B) Hydrodynamic diameter of PDA particles synthesized for 24 h from a 2 mg.mL−1 dopamine solution (50 mM Tris buffer at pH = 8.5) in the presence of HSA at different concentrations (
). The horizontal dashed line corresponds to the saturation absorption at the end of the oxidation kinetics of dopamine. (B) Hydrodynamic diameter of PDA particles synthesized for 24 h from a 2 mg.mL−1 dopamine solution (50 mM Tris buffer at pH = 8.5) in the presence of HSA at different concentrations ( ). Hydrodynamic diameter of PDA particles prepared in the same conditions as previously described and stored in a closed bottle (without refreshed air) for 3 months before characterization by dynamic light scattering (
). Hydrodynamic diameter of PDA particles prepared in the same conditions as previously described and stored in a closed bottle (without refreshed air) for 3 months before characterization by dynamic light scattering ( ). Reproduced from Chassepot and Ball (2014) with authorization.
). Reproduced from Chassepot and Ball (2014) with authorization.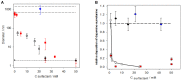
 ), HTAB (
), HTAB ( ), and Triton X-100 (
), and Triton X-100 ( ). The dopamine concentration was 2 mg.mL−1 in the presence of 50 mM Tris buffer at pH = 8.5 in all experiments. The long-dashed lines in the upper part of the figure correspond to the size of the PDA prepared in the absence of surfactant, whereas the short-dashed lines in the lower part of the figure corresponds to the size of the surfactant micelles [measured in the case of SDS (
). The dopamine concentration was 2 mg.mL−1 in the presence of 50 mM Tris buffer at pH = 8.5 in all experiments. The long-dashed lines in the upper part of the figure correspond to the size of the PDA prepared in the absence of surfactant, whereas the short-dashed lines in the lower part of the figure corresponds to the size of the surfactant micelles [measured in the case of SDS ( ) and HTAB (
) and HTAB ( )]. (B) Relative deposition of PDA films as calculated by dividing the thickness of the PDA films, obtained on silicon in the presence of a surfactant, by the film thickness obtained in the absence of a surfactant. The film thickness was obtained by means of single wavelength ellipsometry fixing the complex refractive index of PDA to 1.73 ± 0.02i at λ = 632.8 nm. The deposition of PDA on silicon was investigated in the presence of different concentrations of SDS (___
)]. (B) Relative deposition of PDA films as calculated by dividing the thickness of the PDA films, obtained on silicon in the presence of a surfactant, by the film thickness obtained in the absence of a surfactant. The film thickness was obtained by means of single wavelength ellipsometry fixing the complex refractive index of PDA to 1.73 ± 0.02i at λ = 632.8 nm. The deposition of PDA on silicon was investigated in the presence of different concentrations of SDS (___ ___), HTAB (
___), HTAB ( ), sodium octylsulfate (
), sodium octylsulfate ( ), and Triton X-100 (
), and Triton X-100 ( ). The error bars are calculated from the standard errors on the film thickness produced both in the absence and presence of surfactants. The long-dashed line has the same significance as in (A). Reproduced from Ponzio et al. (2014a) with authorization.
). The error bars are calculated from the standard errors on the film thickness produced both in the absence and presence of surfactants. The long-dashed line has the same significance as in (A). Reproduced from Ponzio et al. (2014a) with authorization.
 ), Films deposited immediately after the end of the 24 h dopamine oxidation (2 mg.mL−1 at pH = 8.5), (
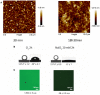
), Films deposited immediately after the end of the 24 h dopamine oxidation (2 mg.mL−1 at pH = 8.5), ( ), film deposited after 3.5 days of aging of the PDA suspension. Inset: Slope of the thickness vs. the number of layer pairs as a function of the aging time of the PDA suspension before deposition of the (PDADMAC-PDA)n film. (B) UV-visible spectra of the (PDADMAC-PDA)n films for n = 2 (black solid line), n = 4 (black long-dashed line), n = 6 (red solid line), and n = 8 (blue solid line). For these experiments, the PDA containing suspension was aged for 24 h before the film deposition. The inset displays the absorbance at λ = 589 nm as a function of the number of deposited layer pairs. The straight line and the dashed lines correspond to a linear regression to the data and to the limits of the 95% confidence interval, respectively. (C) AFM topographies of a (PDADMAC-PDA)10 film prepared using a PDA containing solution aged for 3 days before film deposition. Reproduced from Bernsmann et al. (2010) with authorization.
), film deposited after 3.5 days of aging of the PDA suspension. Inset: Slope of the thickness vs. the number of layer pairs as a function of the aging time of the PDA suspension before deposition of the (PDADMAC-PDA)n film. (B) UV-visible spectra of the (PDADMAC-PDA)n films for n = 2 (black solid line), n = 4 (black long-dashed line), n = 6 (red solid line), and n = 8 (blue solid line). For these experiments, the PDA containing suspension was aged for 24 h before the film deposition. The inset displays the absorbance at λ = 589 nm as a function of the number of deposited layer pairs. The straight line and the dashed lines correspond to a linear regression to the data and to the limits of the 95% confidence interval, respectively. (C) AFM topographies of a (PDADMAC-PDA)10 film prepared using a PDA containing solution aged for 3 days before film deposition. Reproduced from Bernsmann et al. (2010) with authorization.References
-
- Arzillo M., Mangiapia G., Pezzella A., Heenan R. K., Radulescu A., Paduano L., et al. . (2012). Eumelanin buildup on the nanoscale: aggregate growth/assembly and visible absorption development in biomimetic 5,6-dihydroxyindole polymerization. Biomacromolecules 13, 2379–2390. 10.1021/bm3006159 - DOI - PubMed
-
- Ball V. (2010). Impedance spectroscopy and zeta potential titration of melanin films produced by oxidation of dopamine. Colloids Surfaces A. Physicochem. Eng. Asp. 363, 92–97. 10.1016/j.colsurfa.2010.04.020 - DOI
-
- Bernsmann F., Voegel J.-C., Ball V. (2011). Different synthesis methods allow to tune the permeability and permselectivity of dopamine-melanin films to electrochemical probes. Electrochimica Acta 56, 3914–3919. 10.1016/j.electacta.2011.02.028 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

