Snf1 cooperates with the CWI MAPK pathway to mediate the degradation of Med13 following oxidative stress
- PMID: 30175106
- PMCID: PMC6116281
- DOI: 10.15698/mic2018.08.641
Snf1 cooperates with the CWI MAPK pathway to mediate the degradation of Med13 following oxidative stress
Abstract
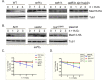
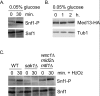
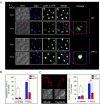
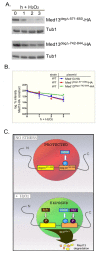
Eukaryotic cells, when faced with unfavorable environmental conditions, mount either pro-survival or pro-death programs. The conserved cyclin C-Cdk8 kinase plays a key role in this decision. Both are members of the Cdk8 kinase module that, along with Med12 and Med13, associate with the core Mediator complex of RNA polymerase II. In Saccharomyces cerevisiae, oxidative stress triggers Med13 destruction, which releases cyclin C into the cytoplasm to promote mitochondrial fission and programmed cell death. The SCFGrr1 ubiquitin ligase mediates Med13 degradation dependent on the cell wall integrity pathway, MAPK Slt2. Here we show that the AMP kinase Snf1 activates a second SCFGrr1 responsive degron in Med13. Deletion of Snf1 resulted in nuclear retention of cyclin C and failure to induce mitochondrial fragmentation. This degron was able to confer oxidative-stress-induced destruction when fused to a heterologous protein in a Snf1 dependent manner. Although snf1∆ mutants failed to destroy Med13, deleting the degron did not prevent destruction. These results indicate that the control of Med13 degradation following H2O2 stress is complex, being controlled simultaneously by CWI and MAPK pathways.
Keywords: AMPK; Cdk8; H2O2 stress; MAPK; Med13; SCFGrr1; Snf1; cyclin C; signal transduction; ubiquitin mediated destruction.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures




Similar articles
-
A complex molecular switch directs stress-induced cyclin C nuclear release through SCFGrr1-mediated degradation of Med13.Mol Biol Cell. 2018 Feb 1;29(3):363-375. doi: 10.1091/mbc.E17-08-0493. Epub 2017 Dec 6. Mol Biol Cell. 2018. PMID: 29212878 Free PMC article.
-
Cdk8 Kinase Module: A Mediator of Life and Death Decisions in Times of Stress.Microorganisms. 2021 Oct 15;9(10):2152. doi: 10.3390/microorganisms9102152. Microorganisms. 2021. PMID: 34683473 Free PMC article. Review.
-
The dual role of cyclin C connects stress regulated gene expression to mitochondrial dynamics.Microb Cell. 2014 Sep 14;1(10):318-324. doi: 10.15698/mic2014.10.169. Microb Cell. 2014. PMID: 28357211 Free PMC article. Review.
-
Cyclin-dependent kinase 8 module expression profiling reveals requirement of mediator subunits 12 and 13 for transcription of Serpent-dependent innate immunity genes in Drosophila.J Biol Chem. 2014 Jun 6;289(23):16252-61. doi: 10.1074/jbc.M113.541904. Epub 2014 Apr 28. J Biol Chem. 2014. PMID: 24778181 Free PMC article.
-
Distinct effects of CDK8 module subunits on cellular growth and proliferation in Drosophila.bioRxiv [Preprint]. 2024 May 3:2024.04.30.591924. doi: 10.1101/2024.04.30.591924. bioRxiv. 2024. Update in: Development. 2024 Dec 1;151(23):dev203111. doi: 10.1242/dev.203111. PMID: 38746212 Free PMC article. Updated. Preprint.
Cited by
-
Cyclin C-Cdk8 Kinase Phosphorylation of Rim15 Prevents the Aberrant Activation of Stress Response Genes.Front Cell Dev Biol. 2022 Mar 31;10:867257. doi: 10.3389/fcell.2022.867257. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433688 Free PMC article.
-
Ubiquitin-proteasome-mediated cyclin C degradation promotes cell survival following nitrogen starvation.Mol Biol Cell. 2020 May 1;31(10):1015-1031. doi: 10.1091/mbc.E19-11-0622. Epub 2020 Mar 11. Mol Biol Cell. 2020. PMID: 32160104 Free PMC article.
-
Cyclin C: The Story of a Non-Cycling Cyclin.Biology (Basel). 2019 Jan 4;8(1):3. doi: 10.3390/biology8010003. Biology (Basel). 2019. PMID: 30621145 Free PMC article. Review.
-
Proteasome granule formation is regulated through mitochondrial respiration and kinase signaling.J Cell Sci. 2022 Sep 1;135(17):jcs259778. doi: 10.1242/jcs.259778. Epub 2022 Sep 7. J Cell Sci. 2022. PMID: 35975718 Free PMC article.
-
The CDK8 kinase module: A novel player in the transcription of translation initiation and ribosomal genes.Mol Biol Cell. 2025 Jan 1;36(1):ar2. doi: 10.1091/mbc.E24-04-0164. Epub 2024 Nov 20. Mol Biol Cell. 2025. PMID: 39565680 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
