Importance of polyphosphate in the Leishmania life cycle
- PMID: 30175107
- PMCID: PMC6116282
- DOI: 10.15698/mic2018.08.642
Importance of polyphosphate in the Leishmania life cycle
Abstract
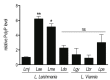
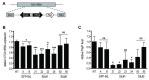
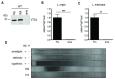
Protozoan parasites contain negatively charged polymers of a few up to several hundreds of phosphate residues. In other organisms, these poly-phosphate (polyP) chains serve as an energy source and phosphate reservoir, and have been implicated in adaptation to stress and virulence of pathogenic organisms. In this study, we confirmed first that the polyP polymerase vacuolar transporter chaperone 4 (VTC4) is responsible for polyP synthesis in Leishmania parasites. During Leishmaniain vitro culture, polyP is accumulated in logarithmic growth phase and subsequently consumed once stationary phase is reached. However, polyP is not essential since VTC4-deficient (vtc4- ) Leishmania proliferated normally in culture and differentiated into infective metacyclic parasites and into intracellular and axenic amastigotes. In in vivo mouse infections, L. majorVTC4 knockout showed a delay in lesion formation but ultimately gave rise to strong pathology, although we were unable to restore virulence by complementation to confirm this phenotype. Knockdown of VTC4 did not alter the course of L. guyanensis infections in mice, suggesting that polyP was not required for infection, or that very low levels of it suffice for lesion development. At higher temperatures, Leishmania promastigotes highly consumed polyP, and both knockdown or deletion of VTC4 diminished parasite survival. Thus, although polyP was not essential in the life cycle of the parasite, our data suggests a role for polyP in increasing parasite survival at higher temperatures, a situation faced by the parasite when transmitted to humans.
Keywords: Leishmania; VTC4; infectivity; life cycle; polyphosphate; temperature stress.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures






Similar articles
-
In vitro infectivity and differential gene expression of Leishmania infantum metacyclic promastigotes: negative selection with peanut agglutinin in culture versus isolation from the stomodeal valve of Phlebotomus perniciosus.BMC Genomics. 2016 May 20;17:375. doi: 10.1186/s12864-016-2672-8. BMC Genomics. 2016. PMID: 27206922 Free PMC article.
-
Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection.J Biol Chem. 2013 Nov 22;288(47):34205-34216. doi: 10.1074/jbc.M113.518993. Epub 2013 Oct 10. J Biol Chem. 2013. PMID: 24114837 Free PMC article.
-
Cryo-EM structure of the polyphosphate polymerase VTC reveals coupling of polymer synthesis to membrane transit.EMBO J. 2023 May 15;42(10):e113320. doi: 10.15252/embj.2022113320. Epub 2023 Apr 17. EMBO J. 2023. PMID: 37066886 Free PMC article.
-
Intracellular growth and pathogenesis of Leishmania parasites.Essays Biochem. 2011;51:81-95. doi: 10.1042/bse0510081. Essays Biochem. 2011. PMID: 22023443 Review.
-
Evolutionary perspective on mammalian inorganic polyphosphate (polyP) biology.Biochem Soc Trans. 2023 Oct 31;51(5):1947-1956. doi: 10.1042/BST20230483. Biochem Soc Trans. 2023. PMID: 37844192 Free PMC article. Review.
Cited by
-
Polyphosphate: A Multifunctional Metabolite in Cyanobacteria and Algae.Front Plant Sci. 2020 Jun 26;11:938. doi: 10.3389/fpls.2020.00938. eCollection 2020. Front Plant Sci. 2020. PMID: 32670331 Free PMC article. Review.
-
Lessons from protozoans: Phosphate sensing and polyphosphate storage in fungi.PLoS Pathog. 2022 Mar 3;18(3):e1010298. doi: 10.1371/journal.ppat.1010298. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35239748 Free PMC article. No abstract available.
-
Molecular characterization of CHAD domains as inorganic polyphosphate-binding modules.Life Sci Alliance. 2019 May 27;2(3):e201900385. doi: 10.26508/lsa.201900385. Print 2019 Jun. Life Sci Alliance. 2019. PMID: 31133615 Free PMC article.
-
An RNA Interference (RNAi) Toolkit and Its Utility for Functional Genetic Analysis of Leishmania (Viannia).Genes (Basel). 2022 Dec 28;14(1):93. doi: 10.3390/genes14010093. Genes (Basel). 2022. PMID: 36672832 Free PMC article.
-
Gene editing and scalable functional genomic screening in Leishmania species using the CRISPR/Cas9 cytosine base editor toolbox LeishBASEedit.Elife. 2023 May 24;12:e85605. doi: 10.7554/eLife.85605. Elife. 2023. PMID: 37222701 Free PMC article.
References
-
- Kornberg A. Inorganic polyphosphate: a molecule of many functions. Prog Mol Subcell Biol. 1999;23:1–18. - PubMed
-
- Kulaev I, Vagabov V, Kulakovskaya T. New aspects of inorganic polyphosphate metabolism and function. J Biosci Bioeng. 1999;88(2):111–129. - PubMed
-
- Brown MR, Kornberg A. The long and short of it - polyphosphate, PPK and bacterial survival. Trends Biochem Sci. 2008;33(6):284–290. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
