Pharmacogenetic Labeling of FDA-Approved Drugs: A Regulatory Retrospective
- PMID: 30175278
- PMCID: PMC6115648
- DOI: 10.1016/j.jacbts.2018.06.001
Pharmacogenetic Labeling of FDA-Approved Drugs: A Regulatory Retrospective
Abstract
The U.S. Food and Drug Administration recently marked 10 years since first updating the labeling for warfarin (often referred to as the "poster child" of pharmacogenomics) to include information regarding the potential impact of CYP2C9 and VKORC1 genetic variation on warfarin dosing requirements and risks. Herein, we opine on the experience updating the warfarin labeling, highlighting more generally the enabling factors and challenges encountered when considering incorporation of pharmacogenomic information into the prescribing recommendations for already approved drugs. We also provide a historical perspective of implemented changes in regulatory policies related to personalized medicine.
Keywords: drug labeling; pharmacogenomics; precision medicine; regulatory perspective; warfarin.
Figures

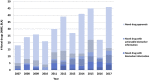

References
-
- Lesko L.J., Zineh I. DNA, drugs and chariots: on a decade of pharmacogenomics at the US FDA. Pharmacogenomics. 2010;11:507–512. - PubMed
-
- Code of Federal Regulations Title 21. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?f.... Accessed March 20, 2018.
-
- Sanderson S., Emery J., Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7:97–104. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

