Thermal pretreatment facilitates recovery from prolonged low-frequency force depression in rat fast-twitch muscle
- PMID: 30175495
- PMCID: PMC6119698
- DOI: 10.14814/phy2.13853
Thermal pretreatment facilitates recovery from prolonged low-frequency force depression in rat fast-twitch muscle
Abstract
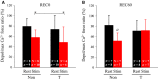
The aim of this study was to examine whether thermal pretreatment can accelerate recovery from prolonged low-frequency force depression. The hindlimbs of thermal treated (T-treated) rats were immersed in water heated to 42.0°C for 20 min (thermal pretreatment). The thermal pretreatment was performed once a day for 5 days before fatiguing stimulation. Intact gastrocnemius muscles were electrically stimulated via the sciatic nerve until force was reduced to ~50% of the initial and dissected immediately [recovery 0 (REC0)] or 60 min [recovery 60 (REC60)] following the cessation of stimulation. Using skinned fiber prepared from the superficial region, the ratio of force at 1 Hz to that at 50 Hz (low-to-high force ratio), the ratio of depolarization (depol)-induced force to maximum Ca2+ -activated force (depol/max Ca2+ force ratio), the steepness of force-Ca2+ concentration curves, and myofibrillar Ca2+ sensitivity were measured. At REC0, the low-to-high force ratio and depol/max Ca2+ force ratio decreased in stimulated muscles from both non- and thermal-treated rats. At REC60, these two parameters remained depressed in non-treated rats, whereas they reverted to resting levels in T-treated rats. Thermal pretreatment exerted no effect on myofibrillar Ca2+ sensitivity. The present results reveal that thermal pretreatment can facilitate recovery of submaximum force after vigorous contraction, which is mediated via a quick return of Ca2+ release from the sarcoplasmic reticulum to resting levels.
Keywords: Calcium release; low-frequency muscle fatigue; reactive oxygen species; sarcoplasmic reticulum.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures




Similar articles
-
Predominant cause of prolonged low-frequency force depression changes during recovery after in situ fatiguing stimulation of rat fast-twitch muscle.Am J Physiol Regul Integr Comp Physiol. 2016 Nov 1;311(5):R919-R929. doi: 10.1152/ajpregu.00046.2016. Epub 2016 Sep 21. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27654397
-
Treatment with EUK-134 improves sarcoplasmic reticulum Ca2+ release but not myofibrillar Ca2+ sensitivity after fatiguing contraction of rat fast-twitch muscle.Am J Physiol Regul Integr Comp Physiol. 2019 May 1;316(5):R543-R551. doi: 10.1152/ajpregu.00387.2018. Epub 2019 Feb 22. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 30794441
-
Contribution of impaired myofibril and ryanodine receptor function to prolonged low-frequency force depression after in situ stimulation in rat skeletal muscle.J Muscle Res Cell Motil. 2015 Jun;36(3):275-86. doi: 10.1007/s10974-015-9409-1. Epub 2015 Feb 20. J Muscle Res Cell Motil. 2015. PMID: 25697123
-
Effects of vigorous isometric muscle contraction on titin stiffness-related contractile properties in rat fast-twitch muscles.Am J Physiol Regul Integr Comp Physiol. 2021 Dec 1;321(6):R858-R868. doi: 10.1152/ajpregu.00189.2021. Epub 2021 Oct 20. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34668430
-
Role of calpain in eccentric contraction-induced proteolysis of Ca2+-regulatory proteins and force depression in rat fast-twitch skeletal muscle.J Appl Physiol (1985). 2017 Feb 1;122(2):396-405. doi: 10.1152/japplphysiol.00270.2016. Epub 2016 Dec 15. J Appl Physiol (1985). 2017. PMID: 27979982
Cited by
-
Mechanisms of eccentric contraction-induced muscle damage and nutritional supplementations for mitigating it.J Muscle Res Cell Motil. 2022 Sep;43(3):147-156. doi: 10.1007/s10974-022-09625-1. Epub 2022 Jul 19. J Muscle Res Cell Motil. 2022. PMID: 35854160 Review.
References
-
- Andrade, F. H. , Reid M. B., and Westerblad H.. 2001. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox‐modulation. FASEB J. 15:309–311. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72:248‐254. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

