Glycopyrrolate/eFlow CS: The First Nebulized Long-Acting Muscarinic Antagonist Approved to Treat Chronic Obstructive Pulmonary Disease
- PMID: 30175596
- PMCID: PMC6357169
- DOI: 10.1177/1060028018798753
Glycopyrrolate/eFlow CS: The First Nebulized Long-Acting Muscarinic Antagonist Approved to Treat Chronic Obstructive Pulmonary Disease
Abstract
Objective: To review the pharmacology, efficacy, and safety of the first nebulized long-acting muscarinic antagonist (LAMA), glycopyrrolate (GLY)/eFlow closed system (CS) nebulizer, approved for maintenance treatment of chronic obstructive pulmonary disease (COPD).
Data sources: A PubMed search was conducted (January 2000 to July 2018) using the following terms/phrases: nebulized glycopyrrolate, inhalation devices in COPD, long-acting muscarinic antagonists COPD, and COPD survey. Retrieved articles were reviewed to identify additional references.
Study selection and data extraction: Primary and review articles on GLY/eFlow CS and other treatment options for patients with COPD were selected.
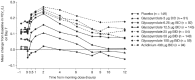
Data synthesis: Guidelines recommend the use of LAMAs, alone or in combination with long-acting β2-agonists, as maintenance therapy for the majority of patients with COPD. With the range of different devices and bronchodilators now available, treatment can be tailored based on individual needs. The eFlow CS nebulizer delivers GLY rapidly over a 2- to 3-minute period and provides bronchodilation within 30 minutes, lasting 12 hours. Phase 2 dose-finding and phase 3 studies demonstrated sustained statistically significant and clinically important improvements in pulmonary function and patient-reported outcomes with GLY/eFlow CS. Relevance to Patient Care and Clinical Practice: GLY/eFlow CS provides a novel, portable, efficient, and rapid drug delivery system.
Conclusions: The recently approved GLY/eFlow CS drug-device combination provides a viable treatment option for patients with COPD, particularly those with conditions that may impair proper use of traditional handheld inhalers.
Keywords: anticholinergics; bronchodilators; chronic obstructive pulmonary disease; drug development and approval; inhalers; pharmaceutical care.
Conflict of interest statement
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2018 report. https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev.... Accessed August 17, 2018.
-
- MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. 2006;332:1202-1204. doi: 10.1136/bmj.332.7551.1202 - DOI
-
- Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1-51. - PubMed
-
- Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66:1-75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

