Bimetallic Nanoparticles in Supported Ionic Liquid Phases as Multifunctional Catalysts for the Selective Hydrodeoxygenation of Aromatic Substrates
- PMID: 30176102
- PMCID: PMC6175319
- DOI: 10.1002/anie.201806638
Bimetallic Nanoparticles in Supported Ionic Liquid Phases as Multifunctional Catalysts for the Selective Hydrodeoxygenation of Aromatic Substrates
Abstract
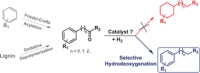
Bimetallic iron-ruthenium nanoparticles embedded in an acidic supported ionic liquid phase (FeRu@SILP+IL-SO3 H) act as multifunctional catalysts for the selective hydrodeoxygenation of carbonyl groups in aromatic substrates. The catalyst material is assembled systematically from molecular components to combine the acid and metal sites that allow hydrogenolysis of the C=O bonds without hydrogenation of the aromatic ring. The resulting materials possess high activity and stability for the catalytic hydrodeoxygenation of C=O groups to CH2 units in a variety of substituted aromatic ketones and, hence, provide an effective and benign alternative to traditional Clemmensen and Wolff-Kishner reductions, which require stoichiometric reagents. The molecular design of the FeRu@SILP+IL-SO3 H materials opens a general approach to multifunctional catalytic systems (MM'@SILP+IL-func).
Keywords: bimetallic nanoparticles; hydrodeoxygenation; iron; ruthenium; supported ionic liquid phases.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Decarboxylation and Tandem Reduction/Decarboxylation Pathways to Substituted Phenols from Aromatic Carboxylic Acids Using Bimetallic Nanoparticles on Supported Ionic Liquid Phases as Multifunctional Catalysts.J Am Chem Soc. 2023 Oct 18;145(41):22845-22854. doi: 10.1021/jacs.3c09290. Epub 2023 Oct 10. J Am Chem Soc. 2023. PMID: 37815193 Free PMC article.
-
Selective hydrodeoxygenation of hydroxyacetophenones to ethyl-substituted phenol derivatives using a FeRu@SILP catalyst.Chem Commun (Camb). 2020 Aug 19;56(66):9509-9512. doi: 10.1039/d0cc03695a. Chem Commun (Camb). 2020. PMID: 32686801
-
Selective Hydrogenation and Hydrodeoxygenation of Aromatic Ketones to Cyclohexane Derivatives Using a Rh@SILP Catalyst.Angew Chem Int Ed Engl. 2020 Jul 13;59(29):11977-11983. doi: 10.1002/anie.201916385. Epub 2020 May 27. Angew Chem Int Ed Engl. 2020. PMID: 32220119 Free PMC article.
-
Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization.Molecules. 2021 May 6;26(9):2728. doi: 10.3390/molecules26092728. Molecules. 2021. PMID: 34066456 Free PMC article. Review.
-
Metal-Support Cooperative Catalysts for Environmentally Benign Molecular Transformations.Chem Rec. 2017 Jan;17(1):4-26. doi: 10.1002/tcr.201600036. Epub 2016 Jul 26. Chem Rec. 2017. PMID: 27455891 Review.
Cited by
-
Acid Catalysis with Alkane/Water Microdroplets in Ionic Liquids.JACS Au. 2021 Jun 28;1(6):786-794. doi: 10.1021/jacsau.1c00107. Epub 2021 May 12. JACS Au. 2021. PMID: 34240079 Free PMC article.
-
Decarboxylation and Tandem Reduction/Decarboxylation Pathways to Substituted Phenols from Aromatic Carboxylic Acids Using Bimetallic Nanoparticles on Supported Ionic Liquid Phases as Multifunctional Catalysts.J Am Chem Soc. 2023 Oct 18;145(41):22845-22854. doi: 10.1021/jacs.3c09290. Epub 2023 Oct 10. J Am Chem Soc. 2023. PMID: 37815193 Free PMC article.
-
Uniformly Dispersed Sb-Nanodot Constructed by In Situ Confined Polymerization of Ionic Liquids for High-Performance Potassium-Ion Batteries.Molecules. 2023 Jul 5;28(13):5212. doi: 10.3390/molecules28135212. Molecules. 2023. PMID: 37446874 Free PMC article.
-
Bimetallic Pd/Sn-based Nanoparticles and their Catalytic Properties in the Semihydrogenation of Diphenylacetylene.ChemistryOpen. 2021 Feb;10(2):296-304. doi: 10.1002/open.202000298. ChemistryOpen. 2021. PMID: 33751864 Free PMC article.
-
Topological engineering of two-dimensional ionic liquid islands for high structural stability and CO2 adsorption selectivity.Chem Sci. 2021 Nov 4;12(47):15503-15510. doi: 10.1039/d1sc05431g. eCollection 2021 Dec 8. Chem Sci. 2021. PMID: 35003578 Free PMC article.
References
-
- Hutchins R. O., Hutchins M. K. in Comprehensive Organic Synthesis, Vol. 8 (Ed.: I. Fleming), Elsevier, Oxford, 1991, pp. 327–362.
-
- None
-
- Corma A., Iborra S., Velty A., Chem. Rev. 2007, 107, 2411–2502; - PubMed
-
- Ruppert A. M., Weinberg K., Palkovits R., Angew. Chem. Int. Ed. 2012, 51, 2564–2601; - PubMed
- Angew. Chem. 2012, 124, 2614–2654;
-
- Besson M., Gallezot P., Pinel C., Chem. Rev. 2014, 114, 1827–1870; - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

